By Stephen Holoviak
This post will attempt to calculate the surface energy of the Pt(111) surface using a slab model and plane wave DFT calculations. Slab models were constructed using different cell parameters, such as number of layers, vacuum spacing and size of supercell. The surface energy for these models was calculated and compared to other models and experimental results.
Introduction:
The existence of a surface on a solid will increase the energy of the atoms at the surface when compared with those atoms in the bulk of the crystal due to the change in coordination atoms at the surface experience. Surfaces can be approximated in DFT calculations through the creation of slab models. The energy of these surfaces, , can be calculated with the following equation, where A is the total surface area of the slab, and E is the system energy of the slab and bulk respectively, and n is the number of atoms in the slab model.
(1)
Experimentally, the surface energy of Pt(111) is found to be (2)
Since the experimental value of the surface energy is fairly small, all calculations were converged to a limit of 5E-3[eV], this limit is depicted on the graphs testing convergence by a red line.
Calculations:
Functional: GGA-PBE(3)
Pseudopotential: “On the fly generation” of ultrasoft psuedopotentals in CASTEP(4) for Pt with the following parameters:
- Core Radius: 2.4[Bhor] ~=1.27[Å]
- 32 electrons in valance with (4f14 5s2 5p6 5d9 6s1) configuration.
All k-points assigned with a Monkhorst-Pack method (5).
1-Layer Slab:
In an attempt to model a platinum surface, a slab model with 1 layer of Pt(111) atoms and 10[Å] of vacuum spacing was constructed.
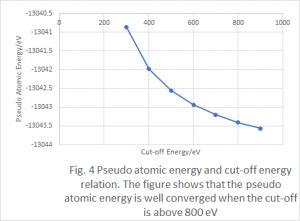
Convergence with respect to the cutoff energy was calculated with a fixed (8x8x1) k-point mesh. The energy of the calculations were then compared to the previous calculation to find the difference. The level of convergence for the cutoff energy was within the previously described limit, which is off of the scale of the graph, indicated by the arrows under the line.
The calculations were found to be well converged for all cutoff energies above 450[eV].
Convergence with respect to the k-point mesh was calculated with a fixed energy cutoff of 450[eV]. The energy of the calculations were then compared to the previous calculation to find an energy difference.
A 1 atom slab of molecules is a very unstable system and not an ideal way to model a surface. Because of this instability changing the number of k-points included in the calculation always changed the energy of the system significantly. There was no consistent value of energy found with sufficient # of k-points.
The number of k-points in the vacuum direction was also increased, generating 8x8x2 and 8x8x4 meshes, to test the effect on the calculations, however there was no effect, changing the slab energy by less than 1E-4[eV].
The amount of vacuum space was also increased from 10[Å] to 20[Å] to test the effect on the calculation, however there was no effect , changing values of slab energy by less than 1E-4[eV].
5-Layer Slab:
A slab model with five layers of Pt(111) and 10[Å] of vacuum space was constructed. This model should represent a solid much better than the 1 layer model.
Convergence for number of k-points and cutoff energy was tested in the same way as the 1 layer model (see figures below). At cutoff of 550[eV] and a k-point mesh of 16x16x1 the calculations were found to be converged to within 5E-3[eV]. The energy of the slab was calculated with these parameters.
The energy of the bulk crystal was then calculated using the same cutoff and a 16x16x16 k-point mesh. Using the calculated energies, the area of the slab, and n = 5 for our slab, the surface energy was found to be 2.4E-2[eV]. This value is much smaller than the experimental value of 0.159[eV].
In order to find the source of this discrepancy, the calculations were repeated for a larger slab with 5 layers, spanning 2×2 and 3×3 unit cells. The surface energies calculated for these cells were similar to the original model, changing only on the order of 1E-3[eV] in final energy.
Changes to the amount of vacuum space were also made to the model and found to have no significant effect on the calculated surface energy, changing the value on the order of 1E-5.
Another attempt to improve the surface energy calculation for this model was to change the functional from the GGA PBE to the LDA CA-PZ(6, 7). It was reported in Vitos (8) that when compared to LDA, GGA predicts surface energies 7-16% lower, and when compared to experimental results predicts values up to 29% lower. The reasoning for this is described as the GGA functional underestimating the exchange energy. When the LDA functional was used for the calculations the surface energy was calculated as 3.4E-2[eV].
This value is a significant improvement over the GGA value, however it is still only a quarter of the experimental value.
8-Layer Slab:
The final attempt to improve the calculated surface energy was done by constructing an 8-layer slab with 10[Å] of vacuum space. The calculations were tested for convergence by the same method as the 1 and 5 layer models. The surface energy calculated with 550[eV] cutoff, 16x16x1 k-point mesh, and LDA CA-PZ functional was 3.4E-2 [eV]. This result is comparable to that of the 5-layer slab.
The vacuum space was changed to 20[Å] in order to test the effects on the calculations. An insignificant shift in energy on the order of 1E-4 [eV] was found.
The supercell size was also changed to 2×2 to test the effects on the calculations. The surface energies calculated for these cells were similar to the original model, changing only on the order of 1E-3[eV] in final energy.
Conclusion:
In order to calculate the surface energy of a metal a common approach is to use slab models. This post found 5-layer and 8-layer slab models resulted in essentially the surface energy. A 1-layer model was found to be a very unstable system and poor physical model. A 10[Å] vacuum spacing was found to be sufficient for all of the slabs tested, and increasing the vacuum spacing had no effect on the calculated energies. It was also demonstrated that the GGA PBE functional underestimates the surface energy more so than the LDA CA-PZ functional.
All models tested significantly underestimated the value of the surface energy when compared to the experimental results. The source of this error is currently unknown.
References:
(1) Sholl, D.S. Steckel, J.A. (2009) “Density Functional Theory: A Practical Introduction.” Wiley, 96-98.
(2) Miedema, A.R. (1978). “Surface energies of solid metals”. Zeitschrift fuer Metallkunde, 69(5), 287-292.
(3) Perdew, J. P. Burke, K. & Ernzerhof, M. (1996) “Generalized Gradient Approximation Made Simple.” 3865–3868.
(4) Clark, S.J., et. al.(2005)”First principles methods using CASTEP”, Zeitschrift fuer Kristallographie 220(5-6) pp. 567-570
(5) Monkhorst, H.J. Pack, J.D. (1976) “Special points for Brillouin-zone integrations.” Physical Review B, 13(12), 5188-5192
(6) Ceperley, D. M. Alder, B. J. “Ground State of the Electron Gas by a Stochastic Method”, Phys. Rev. Lett., 45, 566-569 (1980)
(7) Perdew, J. P. Zunger, A. “Self-interaction correction to density-functional approximations for many-electron systems”, Phys. Rev. B, 23, 5048-5079 (1981).
(8) Vitos L. et. al. (1998) “The surface energy of metals.” Surface Science, 411(1-2), 186-202