by Hepeng Ye
Introduction:
Surface diffusion is a common process for solid materials, and in our case, the Pt adatom could diffuse along the surface of Pt(100). Diffusion is governed by the thermal energy from the environment and the property of such behavior is important for understanding surface phase formation, heterogeneous catalysis and other aspects.
In our case, Pt adatom is hypothesized to undergo a hopping between two adjacent four-fold sites or undergoes concerted substitution. To study the process, a model needs to be built and in our case the model is fairly simple since neither diffusion process has linear interpolation issue, and as consequence, one just need an initial and final two-state model for the transition state study. By connecting the initial and final state of the system, one could locate the TS (transition state) of that process and use the energy barrier from the transition search to further study the kinetic of process.
Experimental:
First cleave the Pt(100) lattice, and create the slab and a five-layer slab of 2X2 super-cell with 5Å vacuum space was set as the starting point. As mentioned the goal of the experiment is to identify the more likely transition process from the two, and in order to get reasonable comparison between the two processes, one need to first optimized the system(slab) to make sure that the super-cell is large enough for boundary conditions and enough vacuum space to prevent interaction from the next periodic slab along the Z-axis. Layers of the slab will not be optimized due to time limit, and based upon experience, five layers should be enough.
For vacuum space optimization, build three slabs with 5Å, 10Å and 15Å vacuum space, and calculate energy with CASTEP, functional used is GGA PBE, pseudo-potential is OTFG-ultrasoft, and all other parameters are set to default (272.1eV energy cutoff, 6x6x1 K-points, core radii is 1.27A with electron configuration to be 5s2 5p6 5d9 and 6s1).
Use the lowest energy vacuum space to continue slab size optimization.
To do this, one will first identify the most stable height of the Pt adatom on the surface. Sitting on top of the fourfold site, it is obvious that the adatom cannot be either too close or too far from the surface where too close to each other there will be repulsion and too far to each other will make Pt adatom isolated from the surface and could interact with the next periodic slab. Consider the short range dispersion, there should be a local energy minimum for the Pt to sit on top of the surface and bring down the system’s energy. So the height is tuned manually by putting the adatom between 1Å to 3Å above the center of the four-fold site.
When Pt adatom transit from one four-fold site to another, the energy for it being reactant should be the same as it being the product. Using this idea, I will separately calculate the energy for Pt adatom sitting at different fourfold sites, for different size of slabs.
The lowest energy height optimized previously will be used, and start with 3×3 super-cell.
As shown in the Figure 2, in 3×3 super-cell, the starting point of Pt, say, at the center, and could diffuse into the nearby fourfold sites. Due to symmetry, the energy barriers for all four direction should be the same, so just consider one of the pathways. In this case, energy of the system is calculated for Pt on the center site and on the edged site.
To get energies of each initial and final state, all calculations are performed with CASTEP, functional used is GGA PBE, pseudo-potential is OTFG-ultrasoft, and all other parameters are set to be the same as above when doing slab-size optimization.
After calculations are done, and comparing the two energies and check the relative difference, and based upon that difference, one will decide whether it necessary to keep using 3×3 super-cell or increase the slab size to 4×4 super-cell or even larger.
Then, in order to find out the transition state, one need to build the initial and final state or the reactant and product state respectively. There are two possible diffusion processes, one is the hopping between two fourfold sites, another is the concerted substitution of Pt adatom with Pt on the top layer. And in the calculations, two assumptions will be made to simplify the problem and reduce computational cost:
- In either process, Pt atom will only diffuse with the closest Pt atom or fourfold site.
- Due to the fact that system used has already optimized its size, and due to the symmetry, only consider Pt adatom diffuse in one direction, instead of four.
Based upon these assumptions, two sets of reactant and product slabs will be built for corresponding diffusion pathways.
After determining the initial and final state of the process, DMol3 TS-search will be used to find the transition state/energy for each process while comparing transitional energy calculated with different search protocols.
Data analysis and discussion:
For the slab size optimization, calculations were performed for 3×3 super-cell where the Pt adatom sits in the fourfold site in the middle, and the fourfold site on the edge, and the energies are close enough to say they are in the same environment.
For the manually adjusted Pt adatom positions, three-point-adjust method is used to iteratively adjust and narrow down the height range and get to the lowest energy and its corresponding height/distance. One could see from the plot that at 1.8Å above the surface, the energy is the lowest.
After that, a 5 layer, 3×3 super slab is applied for the Transition-State study.
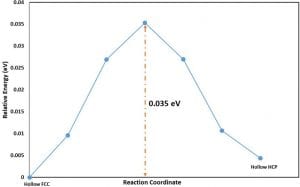
In Castep transition state search, there are some different synchronous transit methods[3], and here, some of these methods were used and were compared their differences. For the fourfold to fourfold hopping, the transition energies for different TS-search protocols are shown in the Table 1 below:

Table 1. Transition energy of hopping between fourfold sites calculated from different TS-search protocol.
For concerted substitution, the transition energy for each TS-search protocol is also listed in the table2.
Different transition searching protocols have different calculation process, but for each process, they are all in agreement with the transition energies are on the same magnitude. While for Pt substitution process, the transition energy barrier is ten times larger than that of hopping process, which makes sense that such process requires breaking Pt metallic bonds with the surrounding Pt atoms and then another Pt atom comes in and forms new bonds, there should be a lot more energy required for such reaction.
Conclusion:
Based upon the energy barrier calculated from the Transition-Search, it is obvious that the concerted substitution has way larger energy barrier than the fourfold sites hopping, which means at same conditions(say same temperature) it will be much less likely for Pt adatom diffuse into the Pt(100) surface and undergoes the concerted substitution process.
There are some experimental setups that could be improved, say the slab I used has 5 layers, which I did not optimize. Also, I stopped at 3×3 supercell size and did not look at 4×4 supercell simply because the energy between two fourfold sites are very close to each other(~0.005 hartree for the total system) for 3×3 supercell, but maybe 4×4 will give even more identical energy, I do not know and maybe someone could try 4×4 disregarding the computational cost.
Reference:
- J.P. Perdew, K. Burke, M. Ernzerhof, Generalized gradient approximation made simple, Phys. Rev. Lett., 77 (1996)
2. J.P. Perdew, J.A. Chevary, S.H. Vosko, K.A. Jackson, M.R. Pederson, D.J. Singh, C. Fiolhais, Atoms, Molecules, Solids, And Surfaces – Applications of the Generalized Gradient Approximation for Exchange and Correlation, Phys. Rev. B, 46 (1992)
3. CASTEP GUIDE, Transition state search task. Date of access: April. 27. 2019
https://www.tcm.phy.cam.ac.uk/castep/documentation/WebHelp/content/modules/castep/tskcasteptss.htm