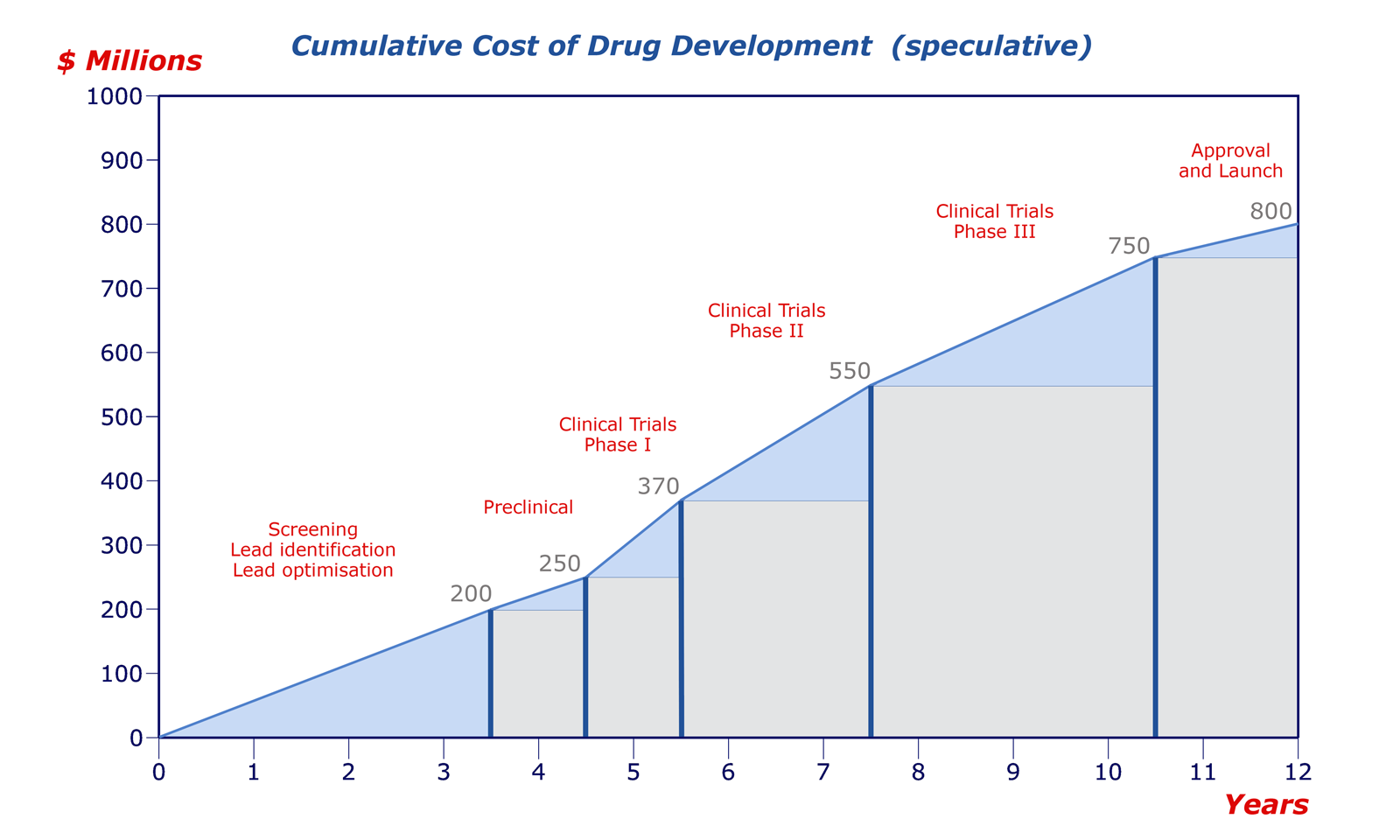
The most costly and time demanding process of the drug discovery timeline is the clinical trials. In fact, everything that happens before this stage is to try and get the drug to “quit early” so that pharmaceutical companies can avoid the hassle of running clinical trials for a drug that is going to fail out in the first phase. Beyond the costs of clinical trials, there are a few different ethical and civic issues surrounding them, some have partial solutions others have been left untouched.
Before we delve a bit deeper into these issues, let’s lay the groundwork for how clinical trials are usually carried out. For pharmaceuticals targeted at everyday ailments and conditions, diabetes for example, there is a very well defined procedure for conducting clinical trials. For conditions like cancer, there are some exceptions.
Phase I tests the medication in healthy individuals to prove that there are no side effects. This phase also helps researchers determine what the proper dose level is and what the lifetime of the drug is in humans. Up until this point, studies have been conducted in animals and other model systems. While researchers can predict what the dosage would be from these studies, it is impossible to tell without seeing it happen in humans. Once a safe dosage range has been determined, the study moves on to Phase II of clinical trials.
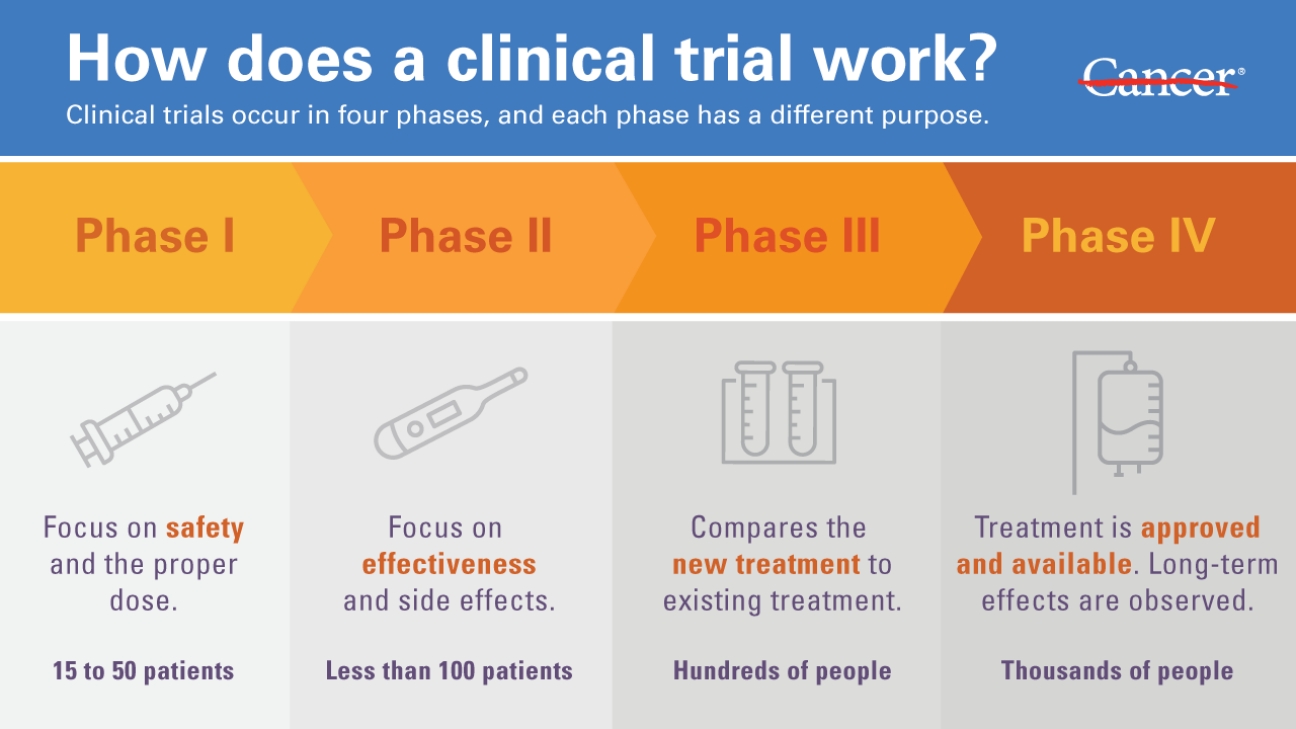
During Phase II, researchers work to determine the efficacy of their drug: does it actually work? Once this phase is over, the company responsible for developing the drug submits a Investigational New Drug Application to the FDA, and once that’s approved, they move on to a much larger and longer Phase III study.
Phase III has the goals of proving what efficacy that was observed during Phase II, on a larger scale and with a much more diverse patient population. If a drug passes through Phase III, then the company responsible then submits a New Drug Application, and waits for FDA approval to begin selling the new prescription.
This entire process has requirements beyond just time and money. It requires a “suitable” patient population. To run clinical trials, you need to have enough patients to do so, and not every condition can provide this.
In the case of rare diseases, it is difficult to develop new medicines for them because the patient populations are so small. In some cases, attempting to run a proper phase I stage of clinical trials would potentially cure the entire patient population.
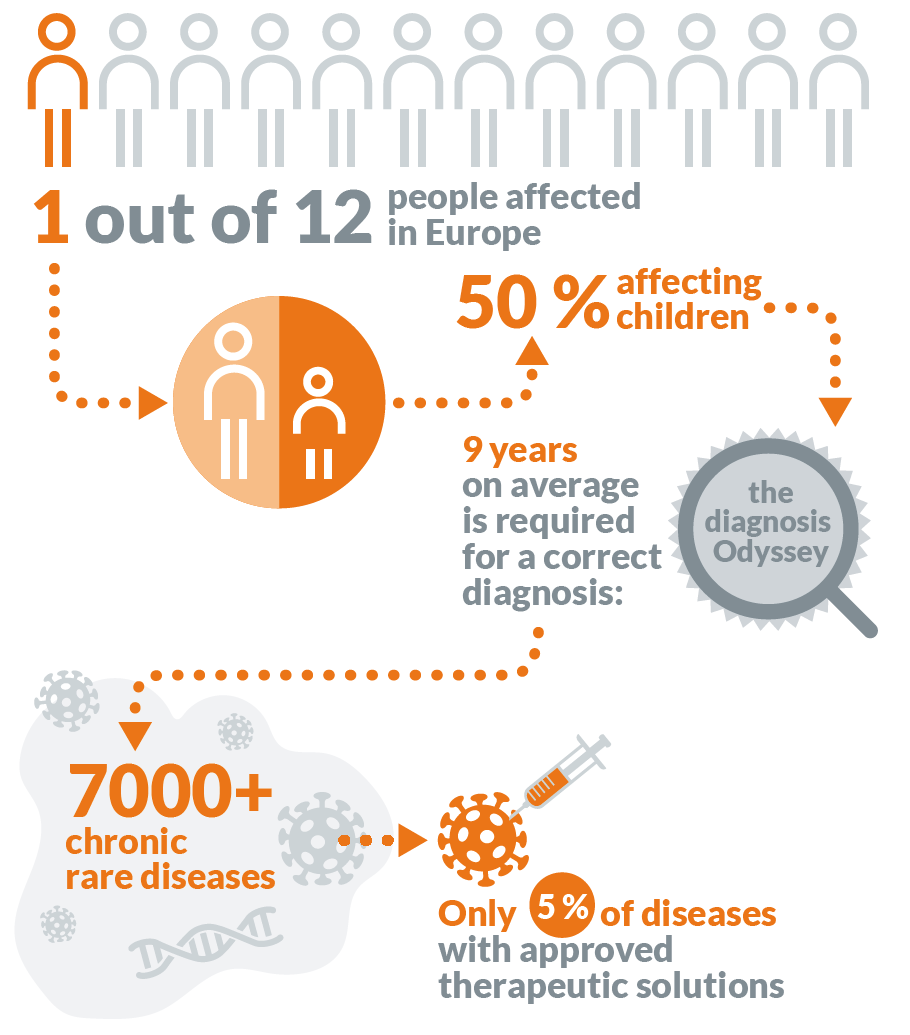
While on paper, this seems like a great thing, in reality it means that few pharmaceutical companies actually get anywhere close to rare diseases. Most drug development companies work by raising money for their disease targets, and it would be near impossible to raise anywhere near the required amount of money for a disease in which the investors were guaranteed not to get their money back (especially considering the success rates are usually so low for normal drug targets).
Beyond rare diseases, there are additional ethical concerns when it comes to running a clinical trial: what do you do for pressing and fatal diseases? how do you decide who can join a clinical trial? how much/do you compensate the patients?
This blog post has already gotten quite long already, so I’ll continue it in the next one.