THERMALLY REGENERATIVE AMMONIA-BASED BATTERY (TRAB)
Considering the depletion of fossil fuels and global climate change, the need for clean and sustainable energy sources is quite evident. A significant potential to obtain clean energy exists from harvesting low-grade thermal energy as electrical power. Low-grade heat (<100 ͦC) use has drawn increasing attention due to its potential for electricity production without the need for additional fuels. A vast amount of low-grade heat is generated from industrial processes and can be collected from solar geothermal sources (Fig. 1). There have been many different methods examined to transform thermal energy into electricity, including solid state devices based on semiconductor materials, organic Rankine cycles, thermoelectrochemical systems (TES), and systems based on salinity gradient energy (SGE).
Figure 1. Various sources of industrial waste heat
Recently, an efficient, inexpensive, and scalable approach was developed at Penn State to generate electrical power from waste heat sources by combining different aspects of the TES and SGE techniques, called a thermally regenerative ammonia-based battery (TRAB). A maximum power density of 115 W m–2 (normalized by the electrode projected area) was produced in a single (first) cycle, with 60 W m–2 produced over multiple successive cycles with electrolyte regeneration (Zhang et al. 2015).
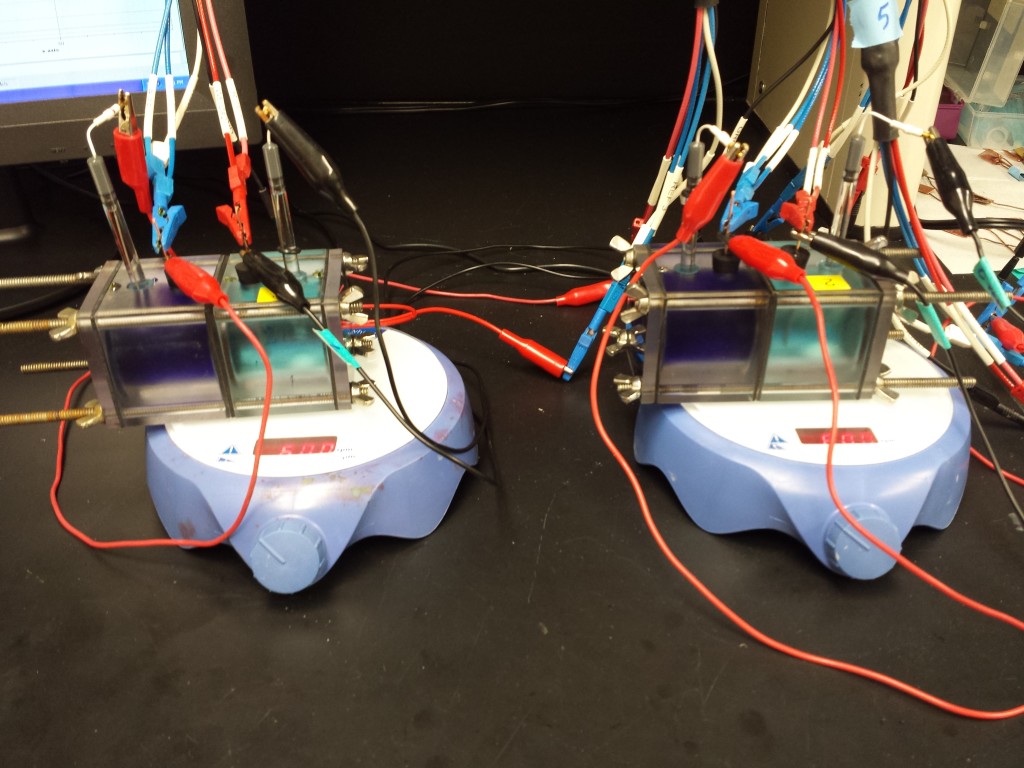
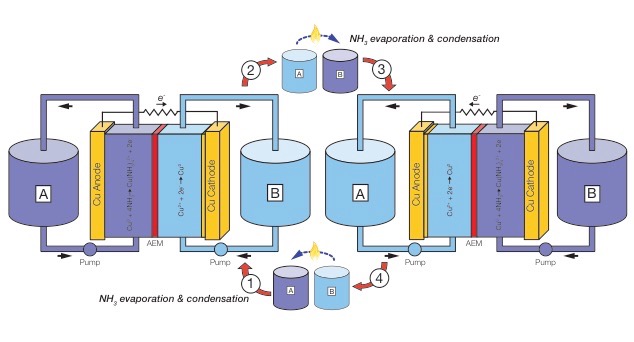
In a TRAB, power is derived from the formation of metal ammine complexes, which are produced by adding ammonia to the anolyte, but not to the catholyte. Ammonia concentration differences between anolyte and catholyte generate a chemical potential, which can be released as electrical current. Only inexpensive materials are used in this process, and the electrodes can be regenerated in successive cycles. In a copper-based TRAB, two copper electrodes are immersed in solutions containing dissolved Cu(II) (copper nitrate), and they are alternately operated as anodes or cathodes in successive cycles (Fig. 2). Copper reduction occurs at the cathode, with copper corrosion at the anode in the presence of ammonia. Low-grade waste heat is used to volatilize the ammonia from the anode, which can be subsequently distilled and added to the other electrode chamber to form a cycle. Currently, we are focusing on improve the heat-to-electricity efficiency by altering the solution chemistry, reactor design, and membrane.Figure 2. Schematic of the TRAB to convert waste heat into electricity