Cellulose is the most abundant biopolymer and a major renewable energy source and biomaterial. It can be enzymatically degraded by cellulases that release cellobiose, which is subsequently split into two glucose molecules that can be fermented into biofuels. However, the partially crystalline structure of cellulose and its interactions with other components of plant cell walls, such as lignin, and xylan, make it resistant to enzymatic degradation. Optimizing cellulase-dependent degradation of lignocellulosic feedstocks can improve biofuels’ cost-effectiveness; however, this requires a complete understanding of cellulose degradation mechanisms by cellulase.
 The exoglucanase Cel7A from Trichoderma reesei is a model cellulase enzyme that degrades the glucan chains of cellulose from their reducing ends. Its mechanism of action has been investigated using traditional biochemical methods, atomic force microscopy (AFM), single-molecule imaging, and optical tweezers, and these results have contributed to quantitative kinetic models of Cel7A activity. Cel7A moves processively at a few nanometers/second, can operate against an external load of 20 pN, and appears stuck in “traffic jams” at high enzyme concentrations. However, some of these results and the ensuing models are contradictory, leaving uncertainty about how Cel7A operates.
The exoglucanase Cel7A from Trichoderma reesei is a model cellulase enzyme that degrades the glucan chains of cellulose from their reducing ends. Its mechanism of action has been investigated using traditional biochemical methods, atomic force microscopy (AFM), single-molecule imaging, and optical tweezers, and these results have contributed to quantitative kinetic models of Cel7A activity. Cel7A moves processively at a few nanometers/second, can operate against an external load of 20 pN, and appears stuck in “traffic jams” at high enzyme concentrations. However, some of these results and the ensuing models are contradictory, leaving uncertainty about how Cel7A operates.

Cellulase TrCel7A
Our current project aims to characterize the mechanisms involved in this hydrolysis process by Cel7A. We have designed our new microscope (SCATirStorm) specifically to measure the mechanisms of Cel7A during the degradation of cellulose at the single-molecule level, such as binding/unbinding rates, unbinding rates, and processivity. This design allows interference reflection microscopy (IRM) and total internal reflection fluorescence (TIRF) microscopy with high temporal and spatial resolution.

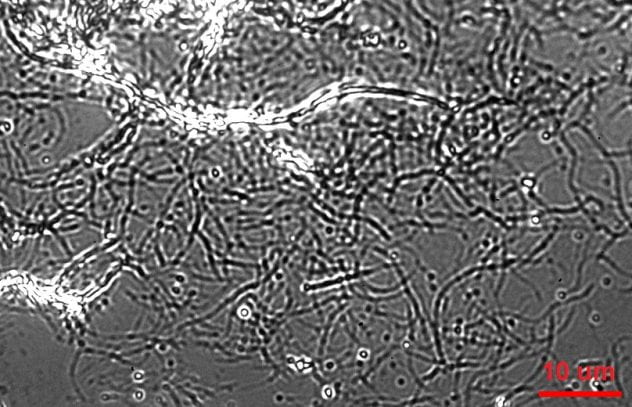
Qdot-labeled Cel7A enzymes reversibly interact with surface-immobilized cellulose
In addition, we also study how Cel7A functions on a variety of cellulose substrates, and under product inhibit conditions, to determine the factors that result in efficient glucose production.
We used IRM to image unlabeled cellulose and tag Cel7A with quantum dots that provide excellent signal/noise and minimal bleaching for visualization by TIRF. Using a custom-built SCATTIRSTORM microscope and Matlab software, we tracked 11,116 Cel7A molecules interacting with bacterial cellulose immobilized on glass coverslips. We found that most Cel7A molecules bind for tens of seconds and dissociate without moving measurably along the cellulose. A subset of the molecules bind and alternate between static and processively moving states. We combined our measurements into a three-state model, whereby Cel7A can bind into or unbind from either a static state or a processive state, respectively. The enzymes occasionally make rapid jumps on the substrate, but we did not observe any evidence that the enzymes diffuse along the substrate while searching for a free-reducing end to digest. These results provide new constraints for modeling the enzymatic mechanism of Cel7A.
Raw movie and PSF fit centers show the static and processing movement of cellulase Cel7A

Raw traces show the processing movement of cellulase Cel7A
This project collaborates with the Ming Tien (BMB) and Charlie Anderson labs (Biology). and is funded by the U. S. Department of Energy (DOE).
Relevant publications:
Integrated multi-wavelength microscope combining TIRFM and IRM modalities for imaging cellulases and other processive enzymes. Daguan Nong, Zachary K Haviland, Kate Vasquez Kuntz, Ming Tien, Charles T Anderson, William O Hancock. Biomedical Optics Express 12 (6), 3253-3264
Nanoscale dynamics of cellulose digestion by the cellobiohydrolase TrCel7A. Zachary K Haviland, Daguan Nong, Kate L Vasquez Kuntz, Thomas J Starr, Dengbo Ma, Ming Tien, Charles T Anderson, William O Hancock. Journal of Biological Chemistry 297 (3)