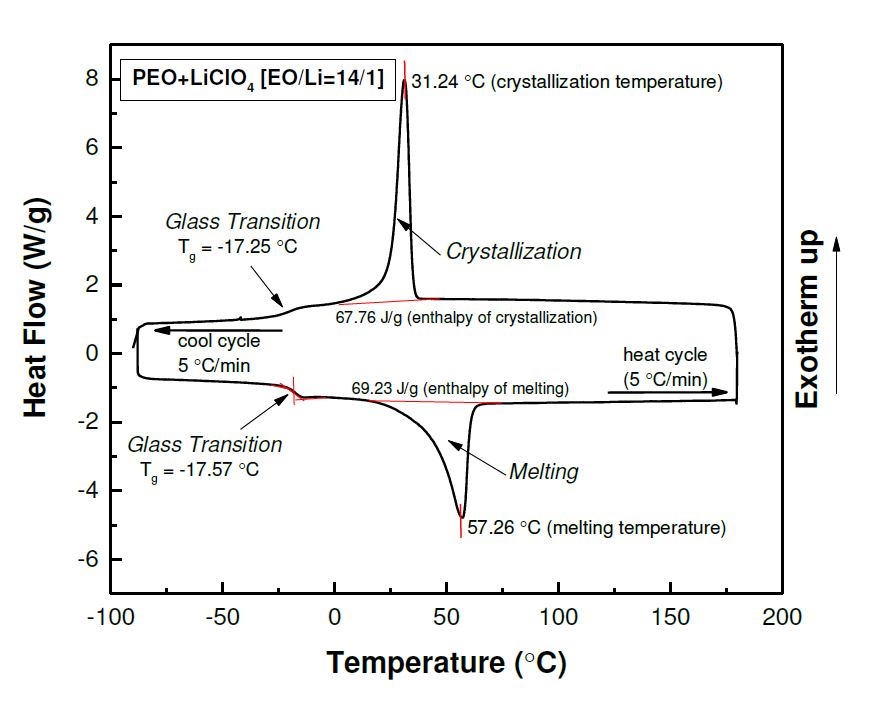
Figure : DSC thermograms of PEO+LiClO4 SPE showing heat and cool cycles at 5C/min. rystallization, melting, and glass transition temperatures are indicated in the Figure. The enthalpy of melting and crystallization are calculated from area under the peak using TA Instruments Universal Analysis software.
We probe the thermal properties of soft matters with Differential Scanning Calorimetry [DSC]. During the cooling and heating scan, when thermal transition occurs, a shift of heat flow supplied to the sample will be observed. The temperature of the heat flow change can be used to determine the melting, crystallization, and glass transition temperature of the material. By integrating the total heat flow during a melting and crystallization process, we can estimate the degree of crystallization of a semi-crystalline sample. The current DSC model we have is TA Instrument Q2000 DSC and the lowest accessible temperature is -80 Celsius.
To apply for time on our DSC in Fenske 129, click here.