Bonus Blog 2: A Nerd’s Chance at Romance
Ciao everyone! Here is this post’s joke:
Did you hear about the new dating site for retired chemists?
It’s called Carbon Dating!
This joke is excellent and I rate it 9.5/10 because I like science jokes. If I’m a single scientist 50 years from now, you’ll definitely find me on this site.
While roasting college romance culture would be entertaining, this week, we’ll be focusing on another type of dating: carbon dating.
According to Britannica, known also as radiocarbon dating, carbon-14 dating is used to determine the age of inorganic and organic materials. This process plays a role across several academic disciplines, from identifying ancient artifacts and understanding the lives of hominids to classifying rocks found in the Earth’s several layers.

checking out some old bonez
Taking a look at the history, “The carbon-14 method was developed by the American physicist Willard F. Libby about 1946. It has proved to be a versatile technique of dating fossils and archaeological specimens from 500 to 50,000 years old. The method is widely used by Pleistocene geologists, anthropologists, archaeologists, and investigators in related fields” (Britannica).
Carbon-14 is an isotope of carbon-12, which is the most abundant type of carbon. With 2 additional neutrons, carbon-14’s radioactivity plays a crucial role in carbon dating and through beta decay (by gaining an electron), will form stable nitrogen-14.

However, with neutron interactions with nitrogen atoms in the environment, carbon-14 can be recreated in Earth’s atmosphere. This is due to increased exposure to cosmic rays, known as high-energy radiation, which increases rates of particle collisions to stimulate chemical reactions.
Carbon-14, or radiocarbon, also comes from carbon dioxide molecules in the atmosphere. After being absorbed by the carbon cycle and plants, this isotope is recirculated among organic matter and reused.
That’s great, but how does carbon dating work?
By exposing matter to carbon-14, it will absorb it, and once the specimen is no longer exposed, it will gradually decrease in concentration of the isotope. Radioactive elements have half lives, meaning that over a certain interval of time, they will half their concentration. From here, the amount of the isotope will decay at a constant and measurable rate. Carbon-14 has a half life of 5,730 ± 40 years, which is insanely long.
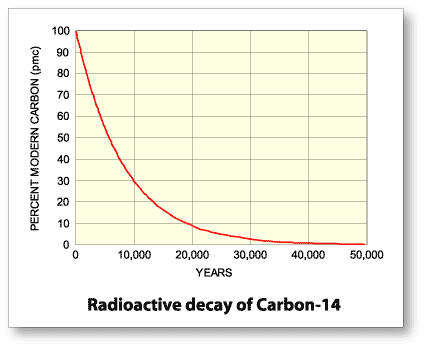
Through spontaneous disintegration, which is basically radioactive decay, carbon-14 will half its concentration after 5,730 years. Because carbon-14 decays at this constant rate, an estimate of the date at which an organism died can be made by measuring the amount of its residual radiocarbon (Britannica).
Carbon-14 is special because of its half life, which is so incredibly long that it gives researchers time to determine how much carbon-14 is present versus the amount of carbon-12 present. This is a great achievement in historical fields, but how else can we use other radioactive isotopes?
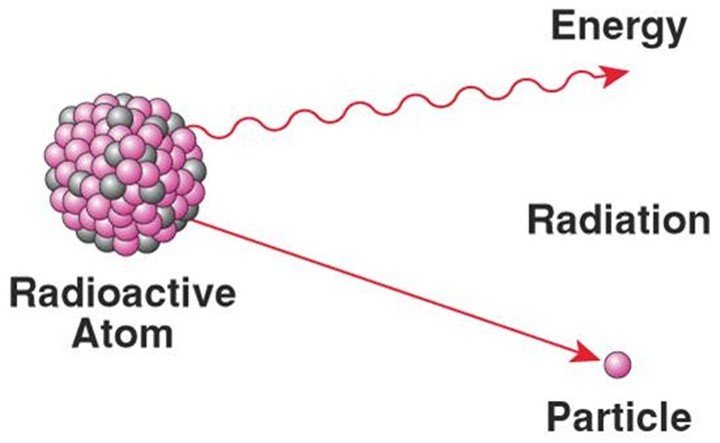
Well, they play an important role in modern medicine, in which half lives can be utilized to track the concentration of certain drugs or treatments in the human body. Very short half lives are desired, which is why Yttrium-90, Iodine-131, Samarium-153, and Phosphorus-32 are used, because who wants chemicals in their body? Not I.
Now, it’s my turn to make like a tree and get out of here. Until next time my jokers!
Reference: “Carbon-14 Dating.” Edited by The Editors of Encyclopædia Britannica,
Encyclopædia Britannica, Encyclopædia Britannica, Inc.,
https://www.britannica.com/science/carbon-14-dating.














/__opt__aboutcom__coeus__resources__content_migration__serious_eats__seriouseats.com__recipes__images__2015__05__20150501-polenta-vicky-wasik-5-018e7507df9940f9b1d9cd0b5f67339b.jpg)