As promised, I will pick up where we left off last week with a discussion of atom clocks and the current definition of the second.
The second is precisely defined as:
“The second, symbol s, is the SI unit of time. It is defined by taking the fixed numerical value of the cesium frequency ΔνCs, the unperturbed ground-state hyperfine transition frequency of the cesium 133 atom, to be 9 192 631 770 when expressed in the unit Hz, which is equal to s^(-1).”
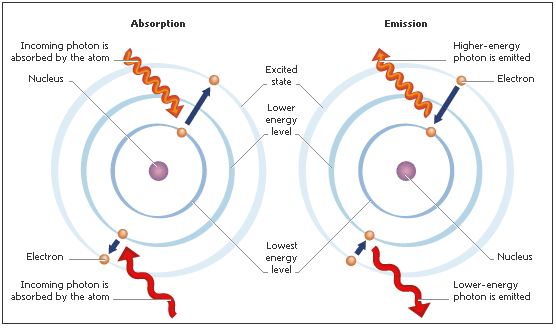
To understand this, one must first understand a little chemistry. An atom can be conceptualized as a positively charged nucleus surrounded by rings of negative electrons. The electrons in the outermost ring (called valence electrons) can essentially jump form their natural ring to a higher-energy ring by absorbing energy. This high-energy state is unnatural though, and the electrons quickly return to the valence shell. The return jump releases the absorbed energy in the form of non-visible light. This emitted light is what is used to define the second.
Returning to the definition, the atom used is specified to be cesium 133 (Cs-133). Cesium is simply an element on the periodic table, and the 133 denotes a certain type of cesium. Restricting the type of atom used ensures that the light being emitted and measured is always the same. The light is further refined with the “unperturbed ground-state hyperfine” part which denotes what electron makes the jump and where. This emitted light travels in a wave form and thus has frequency (the number of waves passing by per second). The frequency of this special cesium light is called the cesium frequency and is given the above value which defines the second.

The actual atom clock device is what is used to carry out the above process. First, the device bombards the sample (the cesium) with radiation. This excites the valence electrons, causing them to jump to the next energy level. Naturally, the electrons soon return to their original level and emit radiation. The atom clock analyzes this emitted light, counting the number of waves passing by. When the correct number of waves has passed, the device reads that one second has elapsed. Newer atom clocks have the added innovation of being “cold clocks,” meaning they operate at very low temperatures. This slows down the motion of the cesium atoms, allowing for more accurate frequency measurements.
Phew, that was a headache. Hopefully I made the definition more accessible to readers. It is not as intuitive as the meter, but certainly less complicated than others. This precise definition for the second is integral in defining the meter, and with these two units, we can begin to branch out into measuring other quantities. By combining the meter and the second, we can measure velocity in m/s and acceleration in m/(s^2). Combining units is part of the beauty of the SI and we will explore more derived units — as they are called — as we discuss the other base units.
Speaking of which, tune in next week for a discussion of the candela, a unit of luminous intensity (aka light).
Again, you blog continues to blow my mind. I never really gave thought to the science and history behind the second and I am constantly learning new things when I read your blog. Like I have said before, I find it amazing that this is your passion and I can see how much you love the subject just by reading your posts. I am looking forward to your posts in the future!
I recently re-learned the chemistry behind electrons moving to different energy levels and you did a good job at explaining it accurately. I would have never thought that the so called “atom clock” would apply chemistry in that way. I am intrigued as to why cesium-133 is used and not some other element. Given its location on the periodic table, it is a large atom, so maybe that has something to do with it.
Wow, I had no idea chemistry was required to measure a second. Like the concept of metrology, chemistry is not my strong suit. Who knew that the release of absorbed energy and non-visible light would be measured for every second? Your definition was simple enough for me to understand though so thank you. Can’t wait to read next week’s blog.