Hydrogen gas can also be produced via fermentation by some bacteria using glucose or cellulose, but the yields are low (a maximum of 4 moles of H2 compared to 12 based on stoichiometry). The Logan Lab at Penn State has done extensive research on fermentation-based hydrogen production, but more recently we have focused on electrochemical hydrogen production in microbial electrolysis cells (MECs). In an MEC, hydrogen gas is produced at the cathode, using exoelectrogenic microorganisms on the anode that convert the organic matter into an electrical current. The voltage that needs to be applied can be as little as 1/10th that needed to split water and make hydrogen gas. The same bacteria that can be used in MFCs to make electricity can be used to generate current in MECs. For a relatively recent and comprehensive review of MFCs and MECs, paper in Science (Logan & Rabaey, 2012)
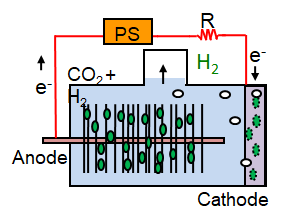 MEC, with additional voltage added using a power source (PS).
MEC, with additional voltage added using a power source (PS).  1.5 L Multi-electrode MEC
1.5 L Multi-electrode MEC  1000 L MEC demonstraed at a winery in Napa, CA
1000 L MEC demonstraed at a winery in Napa, CAIt is possible to have very high hydrogen production yields and energy efficiencies using MECs. For example, in our paper in the Proceedings of the National Academy of Science (PNAS) (Cheng and Logan, 2007, 104(47): 18871–18873), we have obtained hydrogen gas at yields of 2.01-3.95 mol/mol (50-99% of the theoretical maximum) at applied voltages of 0.2 to 0.8 V using acetic acid as the fuel. At an applied voltage of 0.6 V, the overall energy efficiency of the process was 288% based solely on electricity applied (82% from the energy in acetic acid and electrical energy used). The gas production rate was 1.1 m3-H2 per m3 of reactor per day. Direct high-yield hydrogen gas production was further demonstrated using glucose, several other volatile acids (butyric, lactic, propionic, and valeric) and even cellulose at maximum stoichiometric yields of 54 to 91%, at overall energy efficiencies of 64-82%.
Scaling-up MECs has progressed further than that of MFCs, in part due to the simpler 2-phase reaction (protons in water, electrodes) compared to the 3-phase reaction for MFCs (oxygen in air, protons, electrodes). Laboratory scale systems are typically 28 mL cube reactors, such as that shown in the schematic to the left, but we have built larger laboratory systems such as the 2.5 L reactor shown on the left. We also conducted field tests at the Napa Winery in California, and demonstrated effective treatment of winery wastewater using a 1000 L reactor with a net gain in energy relative to the electrical power input, although methane was produced in that system rather than H2 gas. We are currently working on new designs to more effectively convert cellulose fermentation endproducts to H2 gas at high production rates and recoveries, in a project with the National Renewable Energy Laboratory (NREL), in Golden, CO.
Links and notes
This website contains a number of sections to introduce MFCs and other microbial electrochemical technologies (METs). For example, to see slide shows and videos, go to our Presentations page. To find out more about this and other hydrogen and fuel cell research at Penn State, visit the H2E Center webpage. To read more about microbial fuel cells (MFCs), go the the MFC page.