Fundamentally, there are three real forces that can act on an air parcel: Gravity, friction, and pressure-gradient. Gravity and friction probably make the most intuitive sense to you by now because they are heavily stressed in high school physics. As a brief refresher, the force of gravity tends to pull objects, in this case air parcels, towards the center of the Earth, and friction tends to act in the opposite direction of an air parcels’ velocity.
Many times, we only care about horizontal transport of air parcels in the troposphere, and so, gravity is omitted in calculations because it acts perpendicular to the surface of Earth. In a similar manner, since we often only care about this horizontal transport in the upper Troposphere, where interactions with a rugged surface are not common, we also neglect friction. That leaves us with only the pressure-gradient force to worry about.
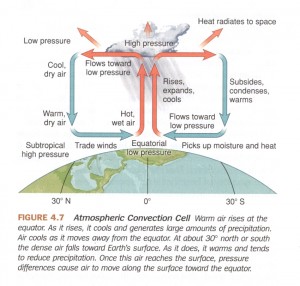
The concept of pressure-gradient force is actually quite simple. Imagine a glass tank consisting of two equally sized chambers separated by a wall. If we then fill the two chambers with unequal amounts of water (i.e. one water level is higher than the other), then we can deduce that the water pressure at the bottom of the chamber with more water in it will be higher than that of the chamber with less water in it. If we now remove the wall between the chambers, what happens? The chamber with more water flows into the chamber with less water until the chambers have an equal water level. Another way of saying this would be that the chamber with higher water pressure flowed towards the chamber with lower water pressure until the water pressures in each chamber reached an equilibrium. This flow from high to low pressure is called the pressure-gradient force.

Here is the same concept of pressure-gradient force presented visually by an arrow.
You would think that, after mastering how to describe these three fundamental forces mathematically, we could explain all phenomena in meteorology. That, however, would be incorrect. We actually require two additional apparent (“fictitious”) forces to enhance our understanding. These correction forces take into account that the Earth is spherical and, more importantly, rotating.
One apparent force is called the Coriolis force. (Perhaps the name sounds familiar to you in your previous Earth science classes. I may go into more details about it in a later blog.) For now, just note that the Coriolis force pulls air parcels ninety degrees to the right of their velocities in the northern hemisphere, as shown below.

The other apparent force is called the centrifugal force. We’ve all experienced its effects when we turn the wheel of our cars. Everything, including ourselves, seems to push away from the center of the circle in which we’re traveling. The same thing is true of air parcels rotating with Earth: They are being pushed away from the centers of the circles in which they are rotating. (For those of you who enjoy physics, the centrifugal force has the same magnitude as the centripetal force, but opposite direction.)
One nice attribute of apparent forces is that they contribute very little when describing motions for small-scale weather systems (i.e. lake-effect snow, sea breezes, tornadoes, etc.). However, when describing the entire motion of the atmosphere and of large-scale weather systems, the Coriolis force, in particular, matters quite a bit. (The centrifugal force, as a whole, is small no matter the size of the system we consider on Earth. Therefore, it is often neglected altogether.)
Let’s take an example of all the information we’ve discovered.
Let’s say that the pressure above the equator is 908mb and the pressure above 30°N is 900mb. If an air parcel is initially at rest above the equator, what will its trajectory appear to look like from an observer on Earth as it moves from higher pressure to lower pressure? (Note: Since we are not moving vertically and we are high up in the troposphere, we can neglect gravity and friction. Thus, the only two forces that we have to consider will be the pressure-gradient and Coriolis forces.)
I think we can all agree that the parcel of air will be attracted to lower pressure at 30°N and, therefore, move northward initially. However, recall that the Coriolis force pulls parcels ninety degrees to the right of their velocities in the northern hemisphere. Therefore, what was once a northerly track becomes a northeasterly track, and what was once a northeasterly track becomes more and more easterly. When a parcel moves due east, notice the pressure gradient force and Coriolis force will balance. (The pressure-gradient force will be pulling the parcel upwards, and the Coriolis force will be pulling the parcel downwards.) An illustration of this force balance is shown below.
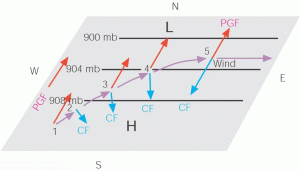
We call this balance between pressure-gradient force and Coriolis force geostrophic flow. It is a fundamental identity, not necessarily a catch-all definition, of how air moves in the troposphere. Nonetheless, we will exploit this special-case situation to describe the “general circulation” of the troposphere, how air moves on average over the entire globe, next time.
So, to summarize, air parcels are generally set into motion by pressure-gradient forces, though friction or gravity could also play a role. These motions are then distorted by the fact that we are rotating on a spherical Earth, and so forces like Coriolis are needed to show how air parcels behave once initially set in motion. A fundamental outcome of the net-effect of all these forces is called geostrophic flow, which essentially states that air parcels will stabilize their directions when the pressure-gradient force is in balance with the Coriolis force. In general, air does not always move in this manner, but it is a decent first approximation.