MVC 9 Awards Gallery
BEST OF SHOW
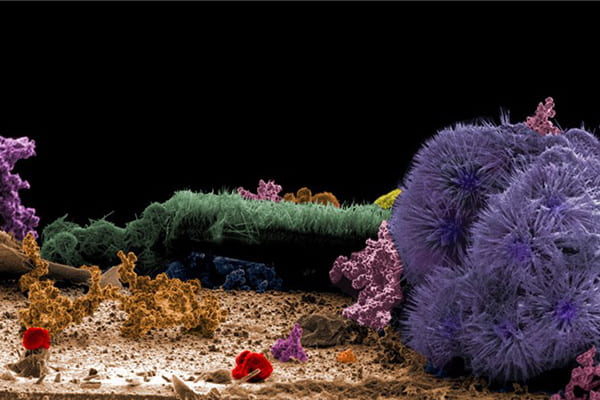
“NANO-SEAFLOOR“
Cameron Holder, Graduate Student, Chemistry
Scientific Process: Hydrothermal synthesis of different morphologies of Co(OH)2 nanowires deposited on FTO electrodes.
SCIENTIFIC CATEGORY
FIRST PLACE
“ADDITIVELY MANUFACTURED 2205 DUPLEX STAINLESS STEEL WITH A KOH ELECTROLYTIC STAINING ETCH“
Andrew D. Iams, Graduate Student, Materials Science and Engineering
Scientific Process: 2205 duplex stainless steel (DSS) offers improved performance compared to traditional stainless steels due to the balanced microstructure of ferrite and austenite. The image presented
shows the as-deposited microstructure of additively manufactured 2205 DSS fabricated using laser based directed energy deposition. The sample was obtained using optical microscopy with a KOH electrolytic
staining etch. Image analysis indicated a ferrite/austenite phase balance of approximately 75/25. No intermetallic phases have been identified.
SECOND PLACE
“POLARIZED OPTICAL MICROSCOPIC IMAGE OF FLOW-INDUCED CRYSTALLIZED NYLON 6/6 AFTER IMPOSING A SHEAR FLOW AT 10 1/S FOR 60 SEC.“
Jiho Seo, Graduate Student, Materials Science and Engineering
Scientific Process: When a semi-crystalline polymer melt is subjected to flow or deformation prior to crystallization, the nucleation rate is accelerated and the crystal morphology changes from spherulites to anisotropic
structures. These phenomena are called flow-induced crystallization (FIC). The flow-induced morphological transformation of Nylon 6/6 was observed using polarized optical microscopy. A Nylon 6/6 sample, which was fabricated
using 16 mm parallel plates with a shear rate of 10 1/s for 60 s at 270 C, exhibited a mixture of anisotropic cylinderites and smaller spherulites at the edge of the disc.
THIRD PLACE
“FORMATION OF GOETHITE FRAMBOIDS DURING SHALE WEATHERING“
Xin Gu, Graduate Student, Geosciences/Geochemistry
Scientific Process: Shale weathering is initiated by pyrite oxidation near water table. Here I present a scanning electron microscopy image showing the microstructure of weathered shale. This rock fragment was collected at
13.7 m deep (water table fluctuation zone) from a borehole at Eel River Critical Zone Observatory in north CA. The unusual morphology of framboidal goethite (aggregates of discrete equant crystals) is similar to framboidal pyite,
which indicates goethite formed through pseudomorphic transformation of pyrite. The platy-like vermiculite growth between goethite crystals documented chlorite weathering in shale. This thin section was imaged by FEI
NanoSEM 630 FESEM microscope with a vCD detector. The horizontal field width is 11.65 µm. Energy dispersive X-ray spectroscopy and Raman spectroscopy were performed to identify minerals.
VISUAL CATEGORY
FIRST PLACE
“LITHIUM DENDRITES WITH GRAND PRISMATIC SPRING
MORPHOLOGY FORMED AT CYCLED LI-ION BATTERY ANODE “
Zhe Liu, Graduate Student, Materials Science and Engineering
Scientific Process: Suppression of Li dendrites formation at anodes during battery cycles is the major challenge in commercializing Li metal anode for next generation high energy density
Lithium-based batteries. This SEM image captures the lithium electrodeposition with a unique Grand Prismatic Spring morphology (The Yellowstone National Park) at battery anode after cycling
SECOND PLACE
“FRACTURED GALAXIES IN SILICON“
Jason Lapano, Graduate Student, Materials Science and Engineering
Scientific Process: Stress cracks nucleate on a black silicon background, like fractures through space. Debris and spots on the surface appear as infinite clusters of stars and galaxies
THIRD PLACE
“FESEM OF FAUJASITE PHASE TRANSFORMED TO ZEOLITE-P“
Taslima A. Zaman, Graduate Student, Chemical Engineering
Scientific Process: Zeolite-P is the synthetic analogue of the gismondine-type (GIS-type) zeolites and is commonly obtained as a side product during faujasite synthesis. This topology of zeolite has a
two-dimensional pore system with two intersecting 8-membered oxygen ring channels of 0.31 × 0.44 nm and 0.26 × 0.49 nm in the [100] and [010] directions, respectively. Zeolite-P has a smaller micropore
size of ~2.9 Å than that of faujasite (~7.4 Å), which makes it useful and valuable in applications for water vapor adsorption and separation of small molecules.
COMPUTATIONAL CATEGORY
FIRST PLACE
“FORMYL ADSORBED ON MODEL COPPER ELECTROCATALYST“
Stephen Weitzner, Graduate Student, Materials Science and Engineering
Scientific Process: Formyl (CHO) adsorbed on a model copper electrocatalyst under applied voltage and embedded in a continuum solvent. Copper is one of the most active catalysts for the electrochemical conversion of carbon
dioxide into renewable hydrocarbon fuels and chemical feedstocks. Yet, in spite of this, carbon dioxide reduction over copper requires sizable overpotentials and can proceed through several overlapping reaction pathways leading to
poor selectivity. In order to assess the origins of these challenges, we determine the thermodynamic stability of reaction intermediates under applied voltage and in contact with a continuum solvent via first principles density-functional
calculations to understand fundamental properties of the electrified metal-water interface. Formyl (COH)—a key catalytic intermediate in the electroreduction of carbon dioxide—promotes the formation of methane over copper surfaces.
Here, formyl is shown adsorbed on a model copper electrocatalyst along with the polarization charges of the continuum solvent model. The polarization charges, shown in orange (positive) and blue (negative), mimic the response of an
explicit solvent in a computationally efficient manner.
SECOND PLACE
“HIGHEST OCCUPIED AND LOWEST UNOCCUPIED
MOLECULAR ORBITALS OF A BLOCK COPOLYMER MOLECULE“
Jason Munro, Graduate Student, Materials Science and Engineering
Scientific Process: This picture shows the highest occupied (top) and lowest unoccupied (bottom) molecular orbitals of a block copolymer molecule, obtained through density functional theory calculations. These provide
information about light absorption and charge transfer characteristics, both of which are vital for photovoltaic applications. This particular polymer exhibits good light absorption, while also effectively separating both holes and
electrons. This, in turn, lowers the intramolecular charge transfer in the material, resulting in the potential for high photovoltaic cell efficiency. Visually, this can be seen by the low amount of spatial overlap between the two orbitals.
THIRD PLACE
“CHARGE TRANSFER DURING PROTON ADSORPTION ON RUO2“
Nathan Keilbart, Graduate Student, Materials Science and Engineering
Scientific Process: Ruthenium dioxide (RuO2) is one of the most prototypical materials for pseudocapacitor electrodes. In order to more fully understand the psedocapacitive properties, density functional theory combined with a
quantum-continuum solvation method to represent the aqueous environment, were used to calculate the electronic structure and redistribution of charge on the RuO2 (110) surface. Charge storage is achieved through quick and
reversible Faradaic redox reactions of the protons at the surface which have minimal lateral interaction. Blue represents the removal of charge while orange is the addition of charge upon adsorption