To understand ozone layer, it would be helpful to know the different layers of the atmosphere. The earth’s atmosphere is composed of many layers, each playing a significant role. The first layer stretching approximately 10 kilometers upwards from the earth’s surface is known as the troposphere. A lot of human activities such as gas balloons, mountain climbing, and small aircraft flights take place within this region.
The stratosphere is the next layer above the troposphere stretching approximately 15 to 60 kilometers. The ozone layer sits in the lower region of the stratosphere from about 20-30 kilometers above the surface of the earth. The thickness of the ozone layer is about 3 to 5 mm, but it pretty much fluctuates depending on the season and geography. 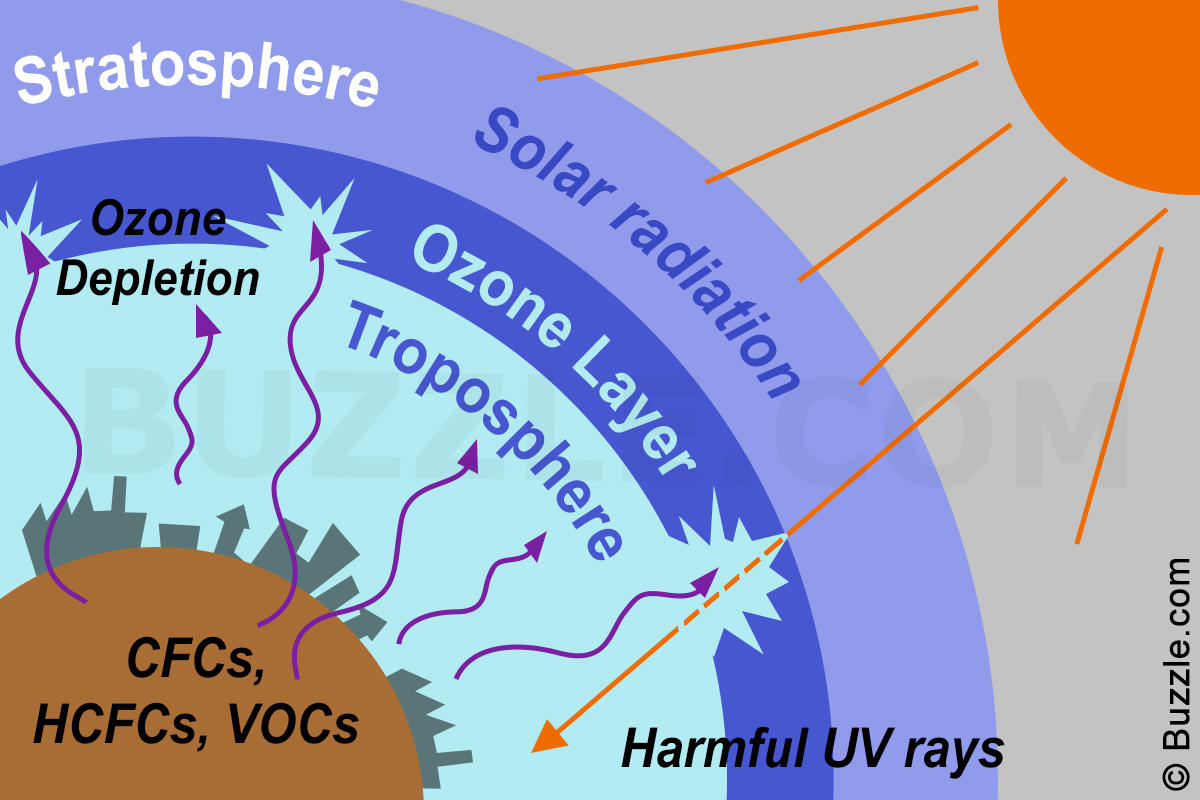
Ozone layer is a deep layer in earth’s atmosphere that contain ozone which is a naturally occurring molecule containing three oxygen atoms. These ozone molecules form a gaseous layer in the Earth’s upper atmosphere called stratosphere. This lower region of stratosphere containing relatively higher concentration of ozone is called Ozonosphere. The Ozonosphere is found 15-35 km (9 to 22 miles) above the surface of the earth.
It comes as a surprise that the same UV rays form the bulk of ozone layer. Ozone is an extraordinary kind of oxygen composed of 3 oxygen atoms instead of the normal 2 oxygen atoms. Ozone layer normally develops when a few kinds of electrical discharge or radiation splits the 2 atoms in an oxygen molecule, which then independently reunite with other types of molecules to form ozone. The ozone layer has been shielding life on planet earth for billions of years, but it’s now being worn out by human activities.
People began to value the importance of the ozone layer when scientists released a research finding suggesting that certain human-made chemicals known as chlorofluorocarbons managed to reach the stratosphere and depleted the ozone via a profound series of chemical reactions. The results of this research study prompted the signing of a global treaty known as the Montreal Protocol in 1973. This treaty helped in the reduction of the production of these harmful human-made chemicals.
These targeted efforts have seen the ozone layer recovering over the past years. The thickness of the ozone layer varies immensely on any day and location. Due to relentless vertical atmospheric air circulation in both the stratosphere and troposphere, the amount of ozone layer shielding humans from strong UV rays can be lesser or greater. In addition, those residing in higher elevations are at risk of UV radiation than those at lower elevations.
So why is the Ozone layer necessary?
An essential property of ozone molecule is its ability to block solar radiations of wavelengths less than 290 nanometers from reaching Earth’s surface. In this process, it also absorbs ultraviolet radiations that are dangerous for most living beings. UV radiation could injure or kill life on Earth. Though the absorption of UV radiations warms the stratosphere but it is important for life to flourish on planet Earth. Research scientists have anticipated disruption of susceptible terrestrial and aquatic ecosystems due to depletion of ozone layer.
Ultraviolet radiation could destroy the organic matter. Plants and plankton cannot thrive, both acts as food for land and sea animals, respectively. For humans, excessive exposure to ultraviolet radiation leads to higher risks of cancer (especially skin cancer) and cataracts. It is calculated that every 1 percent decrease in ozone layer results in a 2-5 percent increase in the occurrence of skin cancer. Other ill-effects of the reduction of protective ozone layer include – increase in the incidence of cataracts, sunburns and suppression of the immune system.
Causes of Ozone layer depletion.
Credible scientific studies have substantiated that the cause of ozone layer depletion is human activity, specifically, human-made chemicals that contain chlorine or bromine. These chemicals are widely known as ODS, an acronym for Ozone-Depleting Substances.
Ozone-Depleting Substances have been proven to be eco-friendly, very stable and non-toxic in the atmosphere below. This is why they have gained popularity over the years. However, their stability comes at a price; they are able to float and remain static high up in the stratosphere. When up there, ODS are comfortably broken down by the strong UV light and the resultant chemical is chlorine and bromine. Chlorine and bromine are known to deplete the ozone layer at supersonic speeds. They do this by simply stripping off an atom from the ozone molecule. One chlorine molecule has the capability to break down thousands of ozone molecules.

Effects of Ozone depletion
- Damage to human health. If the ozone layer is depleted, humans will be overly exposed to strong UV light. Overexposure to strong UV light causes skin cancer, cataracts, sunburns, weakening of immune system and quick aging.
- Devastation to environment. Many crops species are vulnerable to strong UV light and overexposure may well lead to minimal growth, photosynthesis and flowering. Some of the crop species vulnerable to UV light include barley, wheat, corn, oats, rice, broccoli, tomatoes, cauliflower, just to name a few. Forests equally bear the brunt of ozone depletion.
- Threat to marine life. Certain marine life, especially planktons, is greatly impacted by exposure to strong ultraviolet rays. In the aquatic food chain, planktons appear high up. If planktons decrease in number due to ozone layer destruction, the marine food chain would be disrupted in many ways. Also, overexposure of sun rays could reduce the fortunes of fishers. On top of that, certain species of marine life have been greatly affected by overexposure to ultraviolet radiation at their early stage.
Solutions to Ozone depletion
- Refrain from using pesticides. Pesticides are great chemicals to rid your farm of pests and weeds, but they contribute enormously to ozone layer depletion. The surefire solution to get rid of pests and weeds is to apply natural methods. Just weed your farm manually and use alternative eco-friendly chemicals to alleviate pests.
- Use environmentally friendly cleaning products. Most household cleaning products are loaded with harsh chemicals that find way to the atmosphere, eventually contributing to degradation of the ozone layer. Use natural and environmentally friendly cleaning products to arrest this situation.
