The long-term research interest in Dr. Shengyu Yang’s lab is to understand the mechanisms underlying cancer metastasis and developing novel therapeutic strategies in the prevention and treatment of cancer metastasis.
The research in the lab currently focus on two areas related to mitochondrial signaling and metabolism in cancer metastasis.
The first area of research effort is to understand how the actin cytoskeleton remodeling in lung adenocarcinoma promotes metastatic colonization by controlling mitochondrial DNA homeostasis and augmenting mitochondrial oxidative phosphorylation. The second area of research effort is to understand the role of mitochondrial calcium signaling in the control of metabolic stress resistance by activating the Keap1-Nrf2 antioxidant response program in metastatic pancreatic cancer.
Publications:
https://www.ncbi.nlm.nih.gov/myncbi/shengyu.yang.1/bibliography/public/
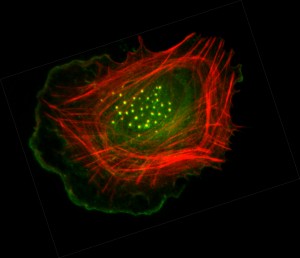
The Metabolic Role of Fascin in Cancer Metastasis
Fascin is the most frequently upregulated actin regulatory protein in metastatic cancers. Fascin overexpression consistently correlates with poor clinical course and shorter survival across different cancer types. As an actin bundling protein, fascin is required for the maximal crosslinking of actin filaments into rigid bundles. It is generally thought that fascin has mechanical roles in promoting cancer cell migration and invasion during metastatic dissemination. There is high interest in developing anti-metastasis therapies targeting fascin. Several small molecule fascin inhibitors have been reported to inhibit cancer cell migration and invasion in vitro, and tumor metastasis in animal models. However, since most metastatic cancer patients already have disseminated cancer cells at the time of diagnosis, blocking dissemination alone might not be sufficient to prevent metastasis in the clinical setting. There is increasing evidence supporting that fascin also promotes oncogenesis, metastatic colonization, anoikis resistance, and cancer cell stemness. These non-canonical functions of fascin are critical for its role in metastatic progression, yet the underlying molecular mechanisms are not clear.
Mitochondria are the powerhouse of the cell. The rewiring of mitochondrial metabolism is crucial for cancer cell stemness and metastatic colonization. The morphology, distribution and homeostasis of mitochondria are dictated by the balance between the constant mitochondrial fission and fusion. The fission of mitochondria is initiated by the pre-constriction of division sites by the mitochondrial F-actin (mtF-actin). The assembly of mtF-actin at the ER-mitochondria contacts is mediated by the ER-anchored actin nucleating protein Inverted Formin 2-CAAX (INF2-CA AX). Spire1C, non-muscle myosin II, and some other actin-regulatory proteins have also been implicated in the assembly and regulation of mtF-actin and the division of mitochondria. However, it is unclear whether and how the dysregulation of the actin cytoskeleton in metastatic cancer cells may regulate mtF-actin remodeling and mitochondrial function. In preliminary studies conducted in non-small cell lung cancer (NSCLC) models, we demonstrated that fascin is essential for the metastatic expansion of disseminated cancer cells and the self-renewal of cancer stem-like cells (CSC). In an effort to reveal the underlying molecular mechanism, we discovered that fascin enhances mitochondrial Oxidative Phosphorylation (OXPHOS) and increases the biogenesis of respiration Complex I by remodeling mtF-actin and regulating mtDNA homeostasis. Our data further indicated that fascin regulates energy stress sensor AMP-activated kinase (AMPK) and Hippo-pathway transcription factor YAP1, which is a crucial mediator for the metabolic control of CSC self-renewal. The mitochondrial regulation by fascin depends on its actin bundling activity, suggesting that small molecule fascin inhibitors could be potentially used to inhibit CSC self-renewal and to prevent metastatic recurrence. We are currently working to define this novel fascin-mitochondria-AMPK-YAP/TAZ signaling circuit in the CSC self-renewal and metastatic expansion in NSCLC. We are also exploring the feasibility to target this circuit to prevent metastatic recurrence.
AX). Spire1C, non-muscle myosin II, and some other actin-regulatory proteins have also been implicated in the assembly and regulation of mtF-actin and the division of mitochondria. However, it is unclear whether and how the dysregulation of the actin cytoskeleton in metastatic cancer cells may regulate mtF-actin remodeling and mitochondrial function. In preliminary studies conducted in non-small cell lung cancer (NSCLC) models, we demonstrated that fascin is essential for the metastatic expansion of disseminated cancer cells and the self-renewal of cancer stem-like cells (CSC). In an effort to reveal the underlying molecular mechanism, we discovered that fascin enhances mitochondrial Oxidative Phosphorylation (OXPHOS) and increases the biogenesis of respiration Complex I by remodeling mtF-actin and regulating mtDNA homeostasis. Our data further indicated that fascin regulates energy stress sensor AMP-activated kinase (AMPK) and Hippo-pathway transcription factor YAP1, which is a crucial mediator for the metabolic control of CSC self-renewal. The mitochondrial regulation by fascin depends on its actin bundling activity, suggesting that small molecule fascin inhibitors could be potentially used to inhibit CSC self-renewal and to prevent metastatic recurrence. We are currently working to define this novel fascin-mitochondria-AMPK-YAP/TAZ signaling circuit in the CSC self-renewal and metastatic expansion in NSCLC. We are also exploring the feasibility to target this circuit to prevent metastatic recurrence.
Happy to share our 2nd paper on the topic of mitochondrial metabolism in lung cancer metastasis. In this paper reported that DGUOK, a mitochondrial nucleotide salvage pathway enzyme, could be a promising target in lung cancer stem-like cells. https://t.co/lRDjBd5QhI
— Shengyu Yang (@shengyuyang1) October 21, 2019
Delighted that our first paper on the role of #mitochondrial #actin filaments in lung cancer metastasis is published @CellReports. We showed that the actin cytoskeleton remodeling in cancer cells is able to promote metastatic expansion by augmenting mitochondrial OXPHOS. 1/n https://t.co/1FgCXAGvVz
— Shengyu Yang (@shengyuyang1) September 10, 2019
Woke up to the news that our DGUOK paper had been accepted. This is the second paper from my lab on mitochondrial metabolism in lung cancer metastasis. The first paper on the subject will be published online @CellReports today. What a great way to start a day!
— Shengyu Yang (@shengyuyang1) September 10, 2019
New grant from the Elsa U. Pardee Foundation
We receive news today that the Elsa U. Pardee Foundation is awarding a new grant to the lab to study a novel Fascin-Nrf2 signaling circuit in lung cancer metastasis and metabolic reprograming. 9/7/2018
Shengchen won a poster award at the Metastasis Research Society Conference
Shengchen and Shengyu attended the Metastasis Research Society Conference in Princeton, New Jershey. Shengchen won a best poster award for his poster on the regulation of lung cancer metastasis and mitochondrial OXPHOS by fascin-mediated remodeling of the mitochondrial actin filament. Congratulations to Shengchen!
July 2018, Shengchen received a DOD Concept Award!
Shengchen won a highly competitive Concept Award from the Department of Defense to study the role of mitochondrial dexoynucleoside salvage pathway in the self-renewal of cancer-stem like cells in non-small cell lung cancer. Congratulations, Shengchen!