
Fig.1. A cartoon of a supported lipid bilayer. The bilayer is separated from the solid support by a thin water layer, which allows the lipids to move within the two-dimensional plane of the bilayer.

Fig.2. A) The left most image is a picture of a polydimethylsiloxane (PDMS) microfluidic device bonded to a glass slide. This device has 5 channels which are 500 microns wide. A 4x magnified view of the channels is shown in the middle image. The right most image depicts fluorescent lipid bilayers in three parallel channels. This image was taken using an epifluorescence microscope.
Metal Ion-Lipid Interactions
A major focus of the Cremer group is investigating the association of transition metal ions with specific lipids in lipid bilayers. Our group found that Cu2+ binds with femtomolar affinity to phosphatidylserine (PS) lipids at basic pH levels (Fig. 3). When Cu2+ binds to PS in a bilayer, it quenches the fluorescence of nearby fluorophores (Fig. 4). (J. Am. Chem. Soc. 134 (2012) 7773-7779 pdf) Currently, we are developing novel assays to measure the binding constants of systems containing various metal ions and lipids. We are also interested in how metal ions change the physical properties of lipid bilayers and determining if metal-lipid interactions are important for apoptosis and lipid flip-flop.
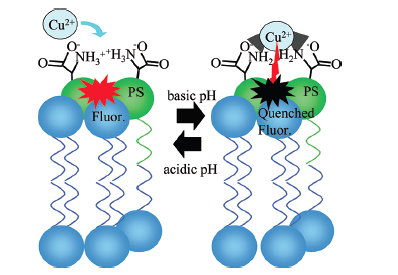
Fig.3. Cu2+ binds to two PS lipids. Cu2+ binds at more basic pH values and unbinds at low pH. Once bound, Cu2+ quenches nearby fluorophore-labeled lipids

Fig.4. (A) Fluorescence images of SLBs in microfluidic channels and (B) line scans of the bilayers when no DOPS (left channel) and 20 mol % DOPS (right channel) was present in SLBs along with 0.1 mol % TRDHPE. The fluorescence is shown to decrease in the presence of Cu2+ only when PS in the membrane and at high pH.
Metal ion-Protein/Peptide-Membrane Interactions
Various proteins or peptides can also bind transition metal ions. For example,amyloid beta peptides (Aβ), which have been explored for their potential involvement in Alzheimer’s Disease (AD), are found to interact with copper, zinc and iron ions. The question then arises as to whether there are interactions between these peptides and lipid membrane bridged by transition metal ions. Furthermore, Aβ aggregates containing these metal ions are often located on lipid domains containing significant concentrations of PS and cholesterol, where these lipids are typically found to be oxidized. Therefore, it is also important to determine if specific lipids serve as reservoirs for metal ions and if proteins or peptides like Aβ can recruit metal ions from membranes and cause redox cycling and lipid peroxidation.

