-
Description of the problem
For this first project, we aim to predict the most-favored crystal structure of Pt and calculate the optimal lattice parameters for these structures.
Usually, the metal crystals can have simple cubic (sc), face centered cubic (fcc), and hexagonal close packed (hcp) structures. In this work, we will firstly examine the optimal lattice parameter for sc Pt based on the energy of bulk Pt, followed by the tests of on the fcc structure. Both optimal lattice parameter a and the ratio a/c will be determined for hcp Pt. Convergence tests will be done with respect to the number of k-points and the cutoff energy for all studies.
The Vienna Ab initio Simulation Package (VASP) is used to perform the periodic DFT calculations,1-3 employing the projected augmented-wave (PAW) pseudopotentials,4,5 as well as generalized gradient approximation with the exchange-correlation functional by Perdew, Burke, and Ernzerhof (PBE).6
-
Simple cubic
The sc structure of Pt is built using the software Material Studio as shown in Figure 1. In each unit cell, there is one Pt atom.


Figure 1. Unit cell of sc Pt Table 1. Results of k-points convergence for sc
Before we can calculate the energy of this whole system, the convergence tests are required with respect to the number of k-points and the cutoff energy. The criteria for choosing the number of k-points and energy cutoff in the following calculation is set to have the energy difference within 0.01 eV.
Firstly, in order to test the convergence of the number of k-points, we initially set the cutoff energy to 400 eV. The results of using number of k-points ranging from 1 to 120 are summarized above in Table 1. Figure 2 shows the trend of bulk energy as well as the computational cost versus the number of k-points.


Figure 2. Bulk energy and computational cost versus the number of k-points for sc
We can see the bulk energy becomes more stable with increased number of k-points while the computational cost keeps increasing. Considering the balance between higher accuracy and cost, the k-points sampling of 14x14x14 is chosen for further calculations.
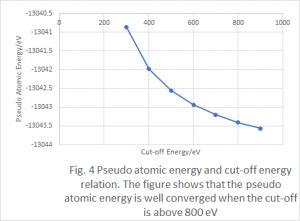
The convergence of the cutoff energy is also tested as summarized in Table 2 with fixed k-points sampling shown above. An interesting thing is that we can see the (pseudo) atomic energy keeps becoming lower while the cutoff energy is increased (later we will see the same behavior for all other structures). Figure 3 shows the relationship of bulk energy and computational costs depending on the cutoff energy.

Table 2. Results of the cutoff energy convergence for sc


Figure 3. Bulk energy and computational cost versus the cutoff energy for sc
Similar as the cases for k-points sampling, the energy becomes stable with increased cutoff energy while the cost keeps increasing. The energy cutoff of 400 eV is chosen for further calculations.
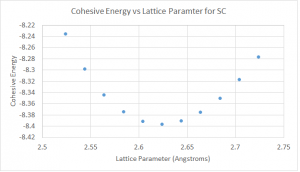
In order to determine the optimal lattice parameter, we vary the lattice parameter to find the one resulting in lowest bulk energy. We firstly do a rough search using step size of 0.1 Å from 1.50 to 3.00 Å. With such rough search, we are able to determine the interval where the optimal value lies in and based on that, we can do a more precious search with step size of 0.01 Å. All the results are shown in Figure 4.


(a) (b)
Figure 4. Bulk energy versus the lattice parameter for sc. (a) for rough search and (b) for detailed search.
From the first plot in Figure 4, we can see that the optimal value is in the interval from 2.50 to 2.70 Å. The detailed search is done in this interval with step size of 0.01Å. Finally, the optimal lattice parameter is determined to be 2.62 Å, which gives the lowest bulk energy of -5.655 eV for the simple cubic structure.
-
Face centered cubic
The search for the optimal lattice parameter of fcc Pt is very similar to the study of sc Pt. The unit cell of fcc Pt is built with Material Studio as shown in Figure 5, containing on Pt atom.


Figure 5. Unit cell of fcc Pt Table 3. Results of k-points convergence for fcc
As we did before, the convergence tests are firstly made and the results on k-points are summarized in Table 2. Figure 6 shows the relationship of bulk energy as well computational time to the number of k-points. Similarly, the k-points sampling of 12x12x12 is finally used.


Figure 6. Bulk energy and computational cost versus the number of k-points for fcc
The same tests on cutoff energy is done as summarized in Table 4 and Figure 7. The cutoff energy we use for further calculations of fcc Pt remains 400 eV.

Table 4. Results of the cutoff energy convergence for fcc


Figure 7. Bulk energy and computational cost versus the cutoff energy for fcc
The search for the optimal lattice parameter is carried out in a similar way, but roughly ranging from 3.10 to 4.30 Å. The results are shown in Figure 8. We can see from the left side of Figure 8 that the optimal lattice parameter lies in the interval from 3.90 to 4.10 Å. Thus, the optimal lattice parameter is determined with the detailed search using step size of 0.01Å. The optimal lattice parameter is 3.97 Å, which gives the lowest bulk energy of -6.097 eV for the face centered cubic structure.


(a) (b)
Figure 8. Bulk energy versus the lattice parameter for fcc. (a) for rough search and (b) for detailed search.
-
Hexagonal close packed
The difference in determining the optimal lattice parameter for hcp Pt is that there will be different optimums for different c/a. So in this section, we will compare the opmital lattice parameter for several potential c/a ratios (in this section, cases of c/a=1.57, 1.60, 1.63, 1.67, 1.70, 1.73 will be studied) and find the most-favored one which gives us the lowest bulk energy among them. Similarly, the hcp unit cell is consturcted using Material Studio as shown in Figure 9 containing 2 Pt atoms.

Figure 9. Unit cell of hcp Pt
The case of c/a=1.60 is chosen to test the convergence. The results are summarized below in Table 5. Figure 10 and 11 show the trends of bulk energy as well as computational cost with respect to the number of k-points and cutoff energy, respectively.


Table 5. Results of the convergence tests for hcp


Figure 10. Bulk energy and computational cost versus the number of k-points for hcp


Figure 11. Bulk energy and computational cost versus the cutoff energy for hcp
Accordingly, the k-points sampling is chosen to be 10x10x6 and the cutoff energy is 400 eV.
The search for optimal lattice parameter is achieved using the same method, but with different c/a values roughly ranging from 2.00 to 3.30 Å. Figure 12 shows the results of rough search for different c/a values.

Figure 12. Bulk energy versus the lattice parameter for hcp (rough search)
We can see that, for the case of c/a=1.56, the optimum lies in the interval from 2.80 to 3.00 Å. For all other cases, the optimal parameter is in the interval from 2.70 to 2.90 Å. The results for corresponding detailed search is shown in Figure 13 below. The final results reveal that the optimal lattice parameter for hcp Pt is 2.76 Å with c/a=1.73, giving the lowest bulk energy of -6.046 eV.

Figure 13. Bulk energy versus the lattice parameter for hcp (detailed search)
-
Conclusion
According to the results above, we know that the optimal lattice parameters for these three different structures are 2.62, 3.97, and 2.72 Å, respectively. Among them, the fcc structure with lattice parameter of 3.97 Å gives the lowest bulk energy of -6.097 eV (-5.655 and -6.046 eV for sc and hcp). The experimental lattice constant is 3.92 Å. Our result is about 1.01% larger than the experimental observation value, which is commonly seen while using PBE functional as PBE tends to overestimate the lattice constant.
Reference
- Kresse, G. and J. Hafner, Ab initio molecular dynamics for liquid metals. Physical Review B, 1993. 47(1): p. 558.
- Kresse, G. and J. Hafner, Ab initio molecular-dynamics simulation of the liquid-metal–amorphous-semiconductor transition in germanium. Physical Review B, 1994. 49(20): p. 14251.
- Kresse, G. and J. Furthmüller, Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Physical review B, 1996. 54(16): p. 11169.
- Kresse, G. and D. Joubert, From ultrasoft pseudopotentials to the projector augmented-wave method. Physical Review B, 1999. 59(3): p. 1758.
- Blöchl, P.E., Projector augmented-wave method. Physical review B, 1994. 50(24): p. 17953.
- Perdew, J.P., K. Burke, and M. Ernzerhof, Generalized gradient approximation made simple. Physical review letters, 1996. 77(18): p. 3865.