Biomaterials Development
Injectable In Situ Polymerizing Bone Grafts “Bone Foam”
Base catalyzed thiol-acrylate Michael addition provides a platform for the development of synthetic composites for use as in situ polymerizing grafts and augments for bone tissue engineering.
Hybrid Semisynthetic ECM Gels
Human adipose is a readily available source of bioactive material for use in the development of grafts and augments. The adipose, which is harvested via lipectomy from consenting donors, would normally be discarded as waste during the course of surgery. After removing resident cells and processing the tissue a potentially usable proteinaceous extracellular matrix (ECM) is obtained containing components such as laminin, fibronectin, and collagen I-IV. A hybrid (semi-synthetic) microenvironment is created combing signaling factors and structures found in naïve adipose tissue and synthetic biomaterials to promote progenitor cell attachment, proliferation and repair of functional tissue.
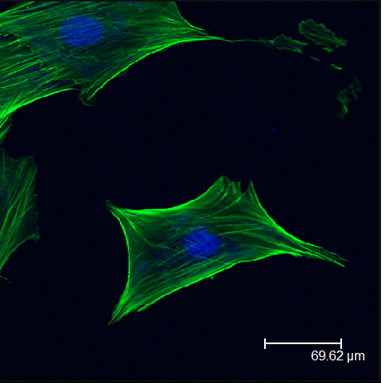
Quasi 3-D Tissue Culture-Cell Sheets
Stackable cell sheets allow for the development of scaffold-free quasi 3-D cell culturing techniques. These structures can more closely represent the in vivo tissue environment allowing us to probe the complex cellular interactions during tissue formation and repair. Stacked stem cell sheets may also provide unique clinical tools for tissue repair and regeneration.
Spatiotemporal Control of Post Translational Gene Regulation
Optically Modulated Gene Regulation
The development of clinically relevant antisense therapeutics for tissue engineering requires improved control over the spatiotemporal delivery of siRNA therapeutics. Photomodulated delivery of siRNA using plasmonic nanoparticles has proven to be an effective tool to control the differentiation and function of progenitor cells both in vitro and in vivo. In this project we aim to develop new optically responsive tools and techniques to spatiotemporally modulate the multiplexed activation of siRNA.

Characterization of SNP-DA-miR148b and miRNA release. (A) Schematic of the release process of caged miR-148b mimics. (B) Hydrodynamic diameters
of SNP, SNP-DA, and SNP-DA-miR148b. (C) UV–vis absorbance spectra of SNP and SNP-DA-miR148b. (D) TEM images of SNP-DA-miR148b. Scale bar, 0.1 μm (E)
Zeta potential of SNP, SNP-DA, SNP-DA-miR148b. (F) miR-148b release profile in the absence or presence of 415 nm LED irradiation.

Rapid and sustained tumor regression in vivo with intravenous delivery of SNP-DA-miR148b. Relative tumor volume post-treatment in different groups. Representative images of the same mouse before and after the treatment.
Targeted Magnetically Modulated Gene Regulation
Magnetically responsive materials provide a convenient means to modulate the spatial and temporal activation of caged therapeutics in deep tissue. This effort aims to develop spatial targeted, magnetically responsive elements for multiplexed gene regulation at depth to provide spatiotemporal control of antisense regulators of tissue repair and regeneration.

Attachment Scheme for MFe2O4 (M = Fe, Co) nanoparticles. Magnetic hysteresis curves for CoFe2O4 and Fe3O4 nanoparticles. Payload release from CoFe2O4 nanoparticles conjugated with FM-TDA linker (in grey), and Fe3O4 nanoparticles conjugated with BIPM-TDA (in black).