Author : Junseok Kim
Introduction
Understanding a diffusion process of an adatom on a metal surface is very important in many catalytic fields, especially for nanocrystal growth. One way for studying the diffusion process is to find a transition state with corresponding activation energy. Regarding to this, we studied Ag atom diffusion on Cu111 surface in this project. Especially, we focused on the hopping of Ag between adjacent threefold sites (fcc and hcp hollow) on Cu111 surface. And, we used Density functional theory (DFT) for all energy calculations since it is a powerful tool for calculating the energy of an atomic surface or slab [1]. Lastly, we determined the transition state for the hopping of Ag atom and the activation energy.
Method
All DFT energy calculations were performed using the Vienna Ab initio Simulation Package (VASP) [2, 3]. For an exchange-correlation functional, the generalized gradient approximation (GGA) – Perdew Burke Ernzerhof (PBE) was used [4]. Projector augmented wave (PAW) was also employed to describe the interaction of ionic core and valance electron [5]. To generate the pseudopotential, 11 valance electrons (3d10 4s1 of the electron configuration) with 2.3 Bohr cutoff radius was used for Cu, while 11 valence electrons (4d10 5s1 of the electron configuration) with 2.4 Bohr cutoff radius was used for Ag.
We employed 3.615 Å from experiment [6] as the lattice constant of bulk Cu for building Cu111 surface. We used a four layered 2 x 2 unit slab for Cu111 surface with fixing two bottom layers and 15 Å of vacuum spacing during the surface optimization and energy calculation, and a single Ag atom was placed on the surface. Due to asymmetric slab, self-consistent dipole correction was included for all energy calculations.
The energy convergence test was carried for the bare Cu111 surface without Ag atom with respect to k-point grid and cut off energy. K-point grid was varied from 5x5x1 to 12x12x1 and cut off energy performed varying the cut off energy from 300 to 550 eV. The convergence test was continued until the difference with respect to the result at the highest k-point grid and cut off energy is less than 0.02 eV.
Then, the geometry optimization for Ag atom on adjacent threefold sites (fcc and hcp sites) of Cu111 was performed with tolerances of 0.01 eV/Å for the force and 10^-6 eV for the energy before determining the transition state. To find the transition state, we used the climbing image nudged elastic band (CI-NEB) method with the reaction tangent force less than 0.05 eV [7]. Also, we used linear interpolation to get 5 intermediate images. To confirm that the obtained transition state is valid, we calculated the vibration frequency for the transition state.
Result
Convergence test
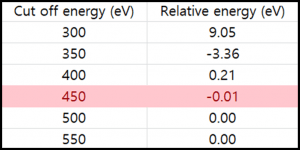
Table 1. The relative total energy of the bare Cu111 surface varying k-point grid (Left) and cut off energy (Right)
Table 1 shows the convergence test for the bare Cu111 surface with respect to k-point grid (Left) and cut off energy (Right). From the convergence test, we obtained the optimal values of k-point grid (8 x 8 x 1) and cut off energy (450 eV), which are used for all calculations.
Geometry optimization of Ag atom for adjacent threefold sites of Cu111
Figure 1. The optimized configuration of Ag on fcc (Left) or hcp (Right) sites on Cu111 surface ; Cu (Orange) and Ag (Light blue).
After the geometry optimization, it is found that there is no significant difference for the Ag-Cu bond length of fcc (2.593 Å) and hcp (2.595 Å) sites as well as their total slab energy, -58.436 eV for fcc site and -58.434 eV for hcp site. Even though the energy difference for Ag on two adjacent sites exists, it is hard to conclude which one is more favorable than the other, since the difference is too small. We may need to calculate the energy for other sites that Ag atom could be also placed on, such as atop or bridge, to determine the most favorable site, which is not important for this project. We used these two optimized states as the end point of transition state for Ag atom diffusion on Cu111 surface.
Transition state and vibration frequencies
Figure 2. Reaction energy profiles using Cl-NEB for the hopping of Ag atom between the adjacent threefold site of surface, fcc and hcp sites.
Figure 3. The configuration (Bridge) of Ag atom on Cu111 surface at the transition state ; Cu (Orange) and Ag (Light blue).
Figure 2 shows the reaction energy profiles for the hopping of Ag atom between the adjacent fcc and hcp sites of Cu111 using Cl-NEB. From Cl-NEB calculation with 5 images along the reaction coordinate, we could find the prospective transition state that has the highest energy. To confirm it is the transition state, we calculated the vibration frequencies for the state, only allowing Ag atom to move during the calculation. A single imaginary frequency (-1.049 THz) was obtained with other two real vibration frequencies, 3.676 and 1.776 THz. As a result, we finally determined the transition state where Ag atom is placed on bridged site of Cu111 surface as shown in Figure 3.
It is found that the activation energy is 0.0568 eV for the hopping from fcc to hcp site, while the reverse activation energy for the hopping from hcp to fcc is 0.0545 eV. The activation energies are quite larger than the reported values (0.023 eV) [8], Shyam’s work (0.0328 eV) and Sharad’s work (0.035 eV). The main reason for these deviations seems because of using a different size of unit cell. We used a relatively smaller size of unit cell (2×2), which leads to a higher surface coverage of Ag than that of others. Even though the coverage effect on Ag atom diffusion on Cu111 surface has not been reported, a high coverage may negatively affect the hopping of Ag on Cu111 and thus lead to the increase in the activation energy [9]. With respect to this, it is worth to note that our value would be a good case for the comparison about the coverage effect on Ag atom diffusion on Cu111. Furthermore, it has been expected that Cu atom can diffuse fast on Cu111 even at room temperature since the activation energy for the self-diffusion (0.03 ~ 0.04 eV) has not much difference with the Boltzmann’s factor (kBT ~ 0.025 eV) at room temperature [10]. Regarding to this, we can also expect that Ag atom would easily diffuse as well as the self-diffusion on Cu111 at room temperature.
Conclusion
In this project, we optimized the adsorption of Ag atom on fcc and hcp sites of Cu111 surface with a very small total energy difference between two sites. And, we successfully determined the transition state for the diffusion of Ag atom on Cu111 surface with Cl-NEB method using DFT. Finally, the activation energy for Ag atom hopping from fcc to hcp site on Cu111 surface is found to be 0.0568 eV, which is not well matched to the reported value but is meaningful. Thus, we believe that this DFT calculation would contribute to understanding the diffusion process of Ag atom on Cu111 surface.
Reference
[1] “Density-Functional Theory of Atoms and Molecules”, R.G. Parr, W. Yang, Y. Weitao (1994), Oxford University Press.
[2] G. Kresse and J. Furthmuller, Comput. Mater. Sci., 1996, 6, 15–50.
[3] G. Kresse and J. Furthmuller, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169–11186.
[4] Perdew, J. P; Burke, K; Ernzerhof, M. Phys. Rev. Lett. 1996, 77, 3865-3868
[5] P. E. Blo¨chl, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 17953–17979.
[6] “Crystallography and Surface Structure: An Introduction for Surface Scientists and Nanoscientists. 2nd edition”, Hermann, K. Wiley-VCH: Berlin, 2016.
[7] G. Henkelman, B.P. Uberuaga, H. Jónsson, the Journal of Chemical Physics, 113 (2000) 9901-9904.
[8] Kotri, E. El koraychy, M. Mazroui, Y. Boughaleb, Surface and Interface Analysis. 2017, 49, 705–711
[9] Inderwildi et al, “Coverage dependence of oxygen decomposition and surface diffusion on
rhodium (111): A DFT study”, J. Chem. Phys. 122, 034710 (2005)
[10] Jian Wang et al, “Diffusion barriers on Cu surfaces and near steps”, 2004 Modelling Simul. Mater. Sci. Eng. 12 1209