Introduction
Zero-point energies (ZPE) are contributions to the total energy of a system due to the vibration of atoms about their equilibrium positions. This energy is exhibited even at 0 K, and is a quantum mechanical effect that can have a significant impact on the energetics of a system. With this being said, many times ZPE is ignored because the computational cost can be prohibitive due to having to calculate all the normal mode frequencies for all atoms in a materials system [1]. But for lighter atoms, such as hydrogen, its vibrational energy can be significant and ZPE can be important to include. In this study, the adsorption of hydrogen at hcp and fcc sites on the Cu(111) surface is explored. These sites are explored as they are the high-coordination sites on the Cu(111) surface making them favorable for adsorption. Calculations that include ZPE and those that do not are compared to predict the importance of including ZPE corrections for this particular system of H adsorbed on Cu(111).
Computational Methods
The density functional theory calculations were carried out using a plane-wave basis set and the projector-augmented wave method as implemented in the Vienna Ab initio Simulation Package (VASP) [2]. The exchange-correlation was approximated using the PBE-GGA functional [3]. Convergence tests gave adsorption energies converged to within 0.002 eV/atom using a cutoff energy of 550 eV, a k-point mesh of 9x9x1 and a vacuum spacing of 10 Angstrom. The Cu(111) slab was modeled using six layers with the bottom two layers fixed and a 2x2x1 supercell was used. Two adsorption sites were considered, those being fcc sites and hcp sites. Figures 1 and 2 show the adsorbed hydrogen on the fcc and hcp sites of Cu(111), respectively. The adsorption energy was calculated using the formula:
(1)
where ,
, and
are the energies of the H atom adsorbed on Cu system, the energy of an H2 molecule, and the energy of the Cu(111) surface, respectively. N is the number of atoms. Using this definition, a more negative binding energy is the more favorable adsorption site. The zero-point energies were calculated using:
(2)
where h is Planck’s constant and corresponds to the vibrational mode that exists for the adsorbed hydrogen atom on the Cu(111) surface.
In all calculations, the value of was -6.771 eV and the value of
was -94.599 eV.
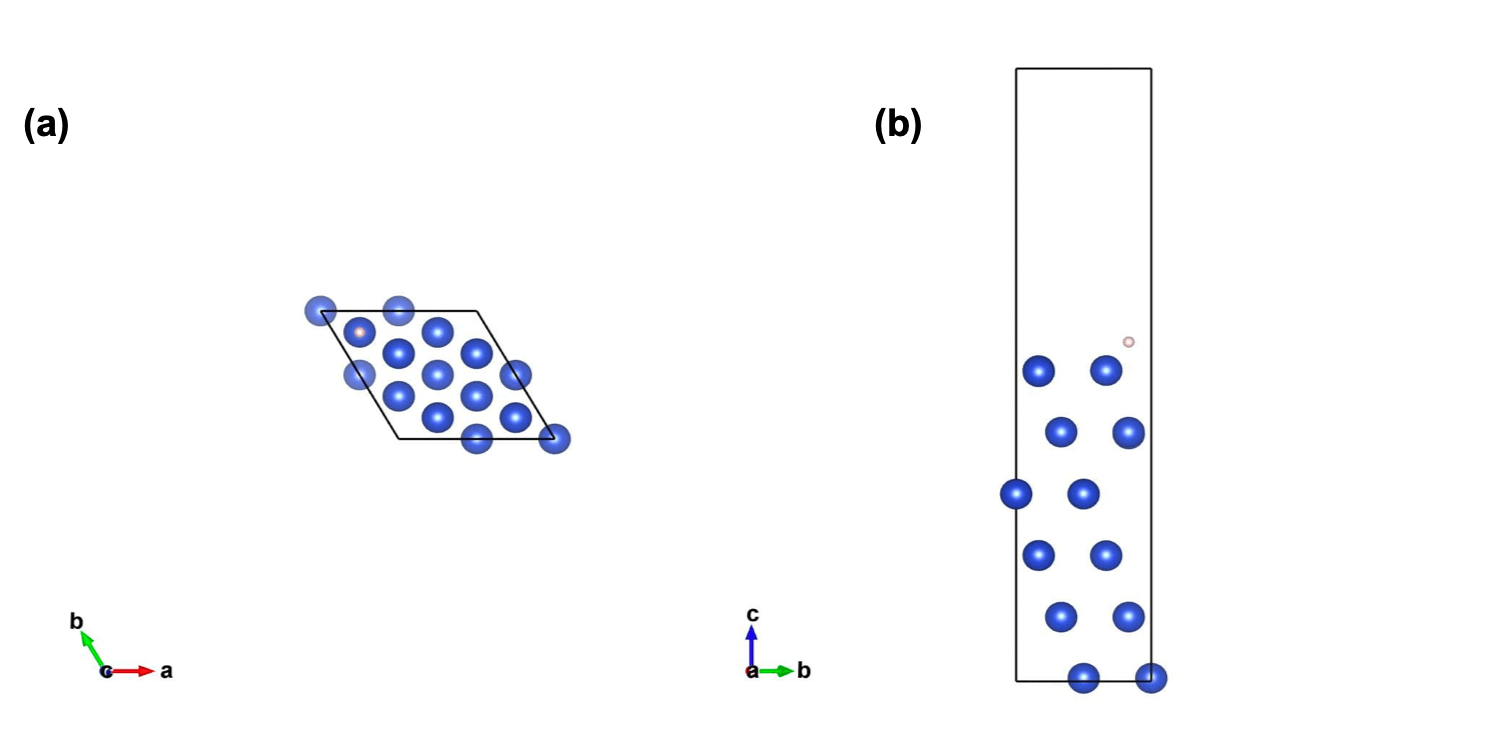
Figure 1: Optimized structure of H atom adsorbed on fcc Cu(111) with (a) top down view and (b) side projection.
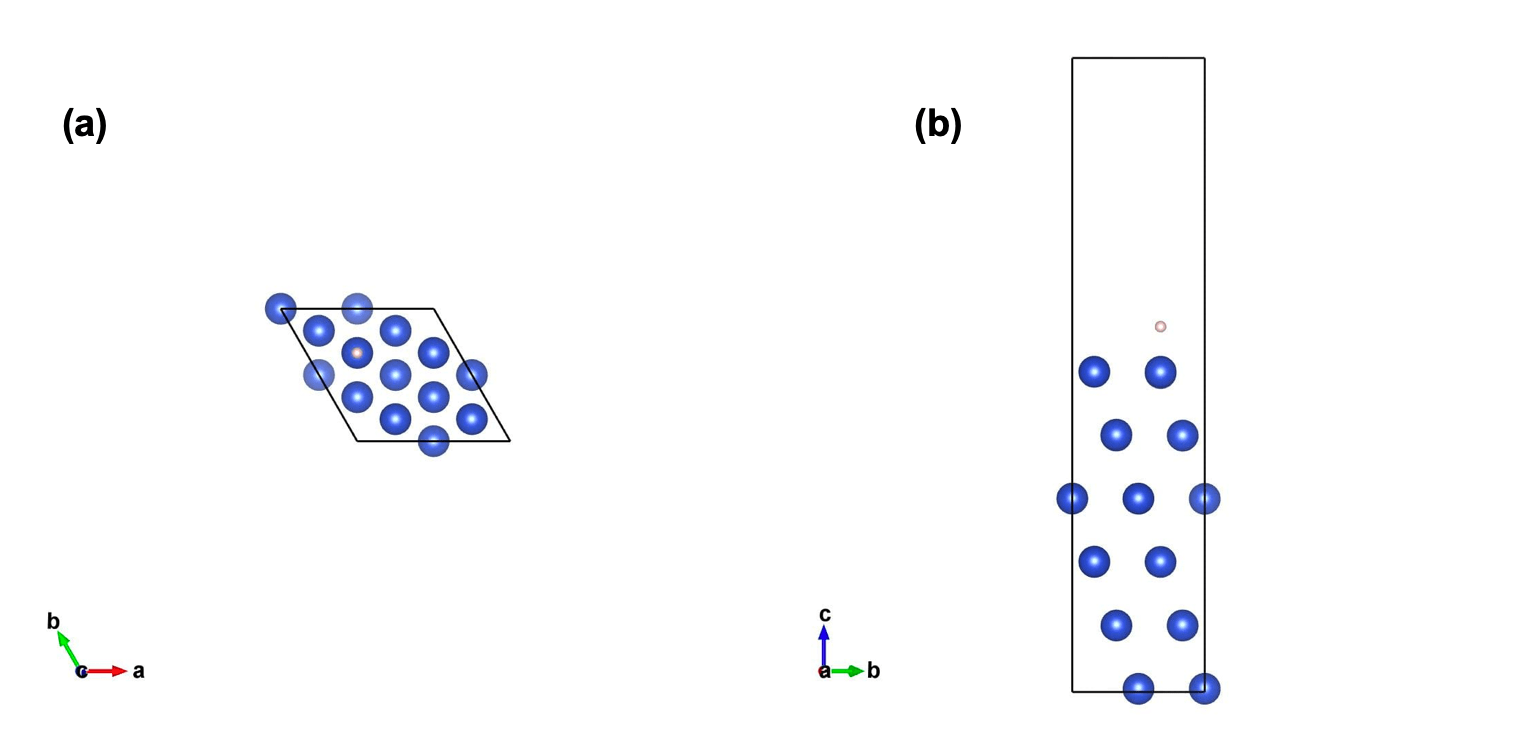
Figure 2: Optimized structure of H atom adsorbed on hcp Cu(111) with (a) top down view and (b) side projection.
Numerical Methods
To calculate the vibrational modes for a collection of atoms, the Hessian matrix must be evaluated. The Hessian matrix is given by
(3)
where is the difference of the atom position from that at the minimum energy configuration. Solving this equation involves invoking numerical approximations. Using the finite-difference approximation, the Hessian matrix can be approximated using the following equation
(4)
Once the Hessian has been determined, the mass-weighted Hessian matrix can be calculated using
(5)
where is the mass of the atom associated with coordinate i. By finding the eigenvalues of the mass-weighted Hessian matrix, the normal mode frequencies can then be calculated using
(6)
where is the wavelength.
Results and Discussion
In the table below lists the adsorption energies for hydrogen on Cu(111) on fcc and hcp sites with and without ZPE corrections and the ZPE values. It is seen from this table that the fcc site is the favorable adsorption site for both situations in which ZPE is ignored and ZPE is included. The difference in adsorption energies between the two sites not including ZPE correction is 0.026 eV/atom and the corresponding value including ZPE is 0.026 eV/atom. These close values of adsorption of H on Cu(111) on fcc and hcp sites are similar to previous calculations by Pang et. al. They defined adsorption energy as a positive value being favorable and obtained values of 2.42 and 2.43 eV for hcp and fcc sites, respectively [4]. In table 2 is listed the adsorption energies for fcc and hcp sites at lower cut-off energy of 450 eV and lower k-point mesh of 5x5x1 while the other parameter is kept at the converged value. While there is a significant difference in the adsorption values, the differences between the adsorption energies of the hcp and fcc sites are similar in all situations.
Sites Adsorption Energy (eV/atom) no ZPE ZPE Adsorption Energy (eV/atom) with ZPE
HCP -0.537 0.073 -0.540
FCC -0.563 0.065 -0.566
| Site | Adsorption Energy (eV/atom) |
|---|---|
| FCC 450 eV cut-off | -0.568 |
| FCC 5x5x1 k-point | -0.555 |
| HCP 450 eV cut-off | -0.542 |
| HCP 5x5x1 k-point | -0.529 |
While the favorable adsorption site doesn’t change while including ZPE corrections, the ZPE is a significant number and it is seen it is important to include in this system. Further considerations that should be considered for future work would be including the effects of the ZPE with the copper atoms in the immediate vicinity of the H atom. The current calculation ignored these effects.
References
[1] Scholl, David S. and Steckel, Janice A. “Density Functional Theory: A Practical Introduction” Wiley (2009).
[2] Kresse, G.; Furthmüller, J. Software VASP, vienna (1999). Phys. Rev. B 1996, 54 (11), 169.
[3] Perdew, J. P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77 (18), 3865.
[4] Langmuir 2007, 23, 9, 4910–4917

