By Charles Bigelow
Abstract
Analysis of two possible methods of diffusion of platinum on a platinum metal surface in the forfold site. The initial and final conditions were optimized and both the hopping and the substitution pathways were analyzed. It was discovered that the hopping mechanism between forfold sites was more energetically favorable than the concerted substitution between the adatom and a surface platinum.
Introduction
Propagation of adsorbed atoms on a metal surface is a chemical phenomena which is of great significance to surface chemistry. Knowledge of this diffusion helps the scientific community to design better catalysts, substrates, and give a better understanding of the underlying mechanics and reaction pathways of a given process.
Metals, in particular, have various ways of migrating across a metallic surface. This is achieved through hopping between different sites on the metal, or through substitution with an atom in the crystal structure itself. These pathways will be analyzed to observe their barrier energy, which will indicate which process is more energetically favorable under normal conditions. This information can be used to help aid in designing pathways for a new catalyst to be produced, as well as to observe how likely catalyst poisoning may occur through substitution.
Figure 1: Platinum Surface with adatom (Top)
Figure 2: Platinum Surface with adatom (side)
Method
Figure 3: 1.5 Layer Slab
A 1.5 layer slab of platinum was generated by cleaving the surface of a bulk sample of platinum along its 100 miller plane. The size of the unit cell was doubled to minimize errors due to periodic boundary conditions; normally the lattice would be tested at various unit cell sizes, however due to computational limitations a larger surface area would not be feasible for the given machine.
A vacuum slab of 13 angstroms was used to ensure that the adatom would not have interactions with the slab above; this is an increase from previous vacuums used earlier in the semester. After generation of the surface, the adatom was generated on the slab at one of the fourfold sites, and its distance optimized from the surface of the lattice manually to save on computational cost. The adatom’s optimal distance from the surface was calculated to be 3.9 angstroms. This was accomplished by varying the distance of the adatom from the surface in the z direction and taking the derivative of the resulting polynomial equation.
An initial and a final slab were generated by moving the adatom to an adjacent site on the platinum surface and placing at the optimal distance. A reaction pathway was generated and 5 steps between initial and final were used to calculate the activation energy. The LDA PWC functional was used to run the calculation using a DND basis set and a basis file of 3.5 [1].
The concerted substitution was done similarly, the only difference was that the initial adatom was associated with the final center lattice atom, and the final adatom associated with the initial center lattice atom. Smearing of 0.005 hartree was implemented for the electron transition of the adatom substitution to remove the discontinuity of the calculation; the hopping mechanism did not require any smearing to run to completion [2].
Results
The most optimal distance was found to be at 3.965 angstroms above the fourfold site, which achieved the lowest equilibrium energy for both the initial and final structures.
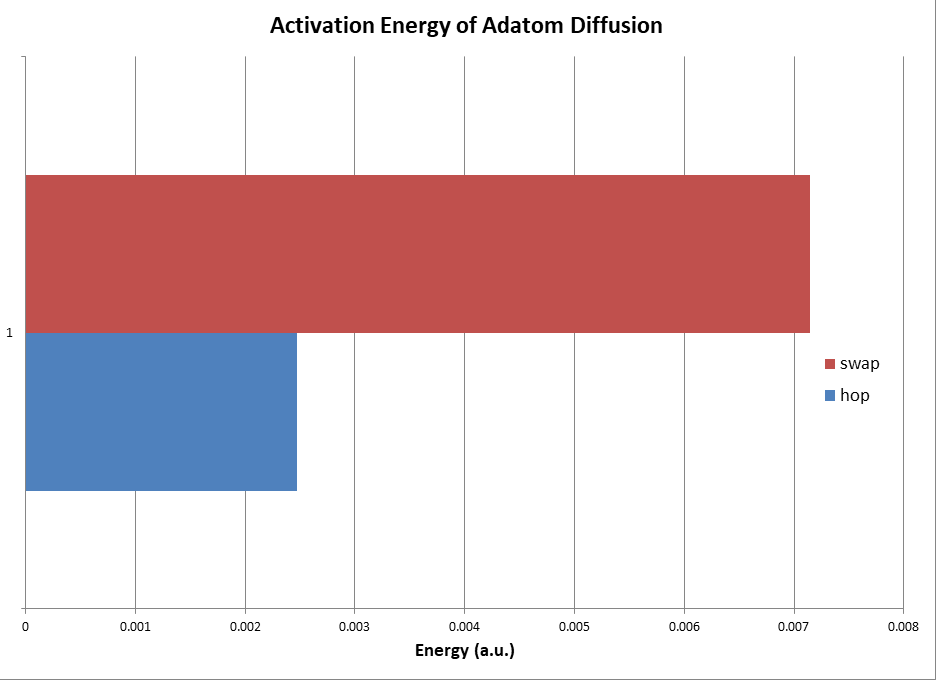
When the two methods of diffusion were analyzed, it was discovered that the hopping substitution had an activation energy of 0.002471 hartree, while the substitution was at 0.007148 hartree.
Conclusion
The hopping mechanism was the lowest energy process in terms of diffusion across the surface of the metal surface, indicating it is the primary method of movement for adatoms on the surface. The substitution method was over double the required activation energy compared to diffusion via hopping; this indicates that this is less likely to occur at a given temperature, however, the energy difference was small enough to allow adatom substitution at a notable rate over time, indicating that the surface would have atoms replaced over an extended time frame. This mechanism is observed in various cases of catalyst poisoning, contributing to the difficulty of effective catalyst design.
URL References
- http://nees.sci.upc.edu.cn/_upload/article/files/39/f5/5460e8894554bd75148145ba414e/188a6221-e993-431c-bf9e-28e3051fd772.pdf
- http://nees.sci.upc.edu.cn/_upload/article/files/39/f5/5460e8894554bd75148145ba414e/188a6221-e993-431c-bf9e-28e3051fd772.pdf