
Perhaps the most bewildering element of the insect body is the wing base. Small wing base sclerites, collectively referred to as pteralia, articulate and connect to each other and to the major thoracic plates in an intricate manner, to serve as the transmission and steering device for the insect flight (they are crucial for the transmission of the motion power generated by the enlarged muscles of the thorax).
The wing of neopteran insects is peculiar: it can be folded above the body in the resting (not flying) position in a way that substantially decreases the body width. Decreasing body width by folding “appendages” is a common phenomenon that is not only favored by nature (e.g. birds and bats fold their wings when not in use or beavers folding fore legs in while swimming) but also by technology (car mirrors or airplane wings or solar panel arrays for space satellites). Folding appendages that are not in use is advantageous for multiple reasons: the body can fit into small places (just think of parasitoid wasps, developing in obscure, size-limited environments, such inside a cockroach ootheca) and the exposed body surface to predators is decreased (compare the habitus of mosquitoes and dragonflies). There is little doubt that the key innovation that led to the evolutionary success of neopterans is wing folding and that this phenotype is largely controlled by the geometry of wing base sclerites.
As being the most famous (or at least most studied) example of epithelial flattened evaginations (see smart box below), the dorsal and ventral epithelial layers of the insect adult wing are, for the most part, fused to each other.
| Box 1. Flattened evaginations of the insect cuticle The insect wing is one of many kinds of flattened evaginations of the single cell layered insect skin (epithelium). These evaginations flatten (usually dorsoventrally) gradually during the course of the adult development until their two flat surfaces almost completely fuse to each other. 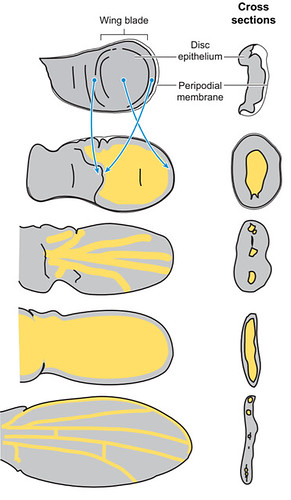 Unfused areas of these evaginations (separated areas marked with yellow and fused with grey in Figure 1.) are usually elongate, narrow regions retained for supplying the more remote (distal) regions of the evagination (e.g. the wing veins marked with yellow lines on the above image or the wing vein-like structures marked as ws on the pronotal flattened evagination of a true bug). |
The two epidermal layers of the wing remain separated only along tubular wing veins and at a relatively narrow, proximal region close to the wing hinge (marked as a red, translucent line in Fig 2; fold line along which the wing is flapped relative to the thorax). While elongate, tubular wing veins “feed” living cells (e.g., sensory neurons) in the distal wing regions, lack of epithelial fusion along the wing base is most likely related to keeping the wing base flexible enough for its desired flapping and folding functions. Fused areas of the wing blade are well sclerotized and rigid, which is not surprising since these regions, the wing cells (not to be confused with the basic functional unit of all life!), provide the effective surface for the flight. In the images below, level of sclerotization or “hardness” of the insect cuticular regions correlates to the wavelength of its autofluorescence: more sclerotized regions appear red/brown, while soft structures are green).
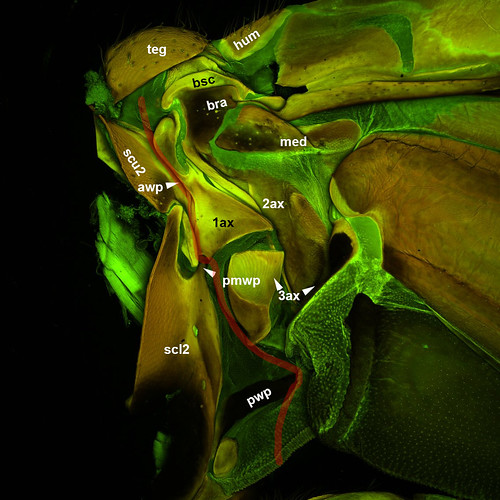
Three wing base sclerites articulate with the dorsal and lateral thoracic plates along the wing hinge: The first axillary is in contact with a lateral projection of the mesoscutum (awp) and an anterior projection of the mesoscutellum (pmwp), the third axillary (3ax) is connected to a more posterior mesoscutellar projection (pwp; this projection is separated in numerous cases from the mesoscutellum) and the second axillary (2ax) to a process on the lateral thoracic wall, the pleuron (plwp).
Besides these articulating wing base sclerites the wing base contains 5 other sclerites: the tegula (teg), the humeral plate (hum), the median plate (med), the basiradiale (brad; a sclerotized proximal portion of the radial vein that is continuous in numerous cases with the second axillary), the basisubcostale (bsc; a sclerotized proximal portion of the subcostal vein that is connected via a ligament to the first axillary) the subalare (sa). Muscles connected to the latter two sclerites control changes in wing kinematics and allow insect specimens to maneuver rapidly thus usually referred as steering muscles.
Since the epithelial layers are unfused at the wingbase, we can classify and readily homologize the pteralia not only based on their relative position to the wing hinge and the above mentioned three articulations but also by their location either on the ventral or dorsal wing membranes. Most of the sclerites mentioned above are located on the dorsal epithelial layer (1st, 3rd axillary, median plate, tegula, humeral sclerite, basisubcostale and the posterior notal wing process). Ventral sclerites are the basalare and the subalare. There are, however, two sclerites that are represented both on the dorsal and ventral wing epithelia: the basiradiale and the second axillary.
This is a really interesting property of a wing base sclerite. How can be something represented on both the dorsal and ventral membrane in a region that is actually lack of epithelial fusion? During my studies of apocritan Hymenoptera wing bases I found that these two regions are always fused in adults. Moreover they are fused in early adult stages in Bombus and in Vespula, where the two wing layers are still separated. The second axillary is not only represented on both wing epithelial layers, but also is articulated and connected via ligaments to both dorsal and ventral components (first and third axillary on teh dorsal layer and the pleural wing process and the basalare on the ventral layer).
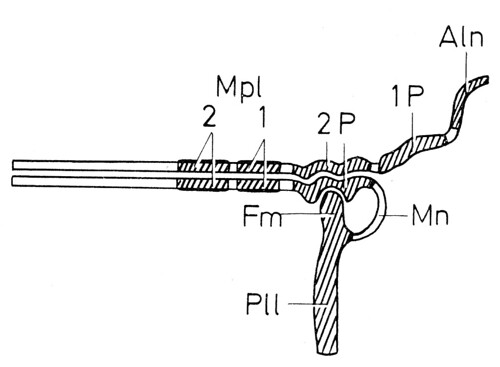
Is it possible that the second axillary (and the basiradiale) are represented by two unfused, dorsal and ventral sclerites an early stage of insect evolution? Although this idea was nicely illustrated in Seifert’s magnificent Entomologisches Praktikum (Georg Thieme Verlag, Stuttgart, 1975)—second axillary (2P) is represented by a ventral and a dorsal sclerites—I was not able to find a single example for this, I guess, ancestral state.
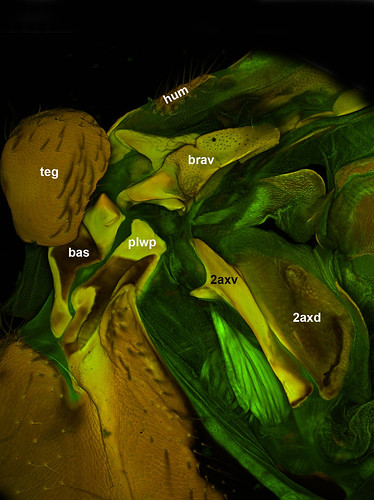
That is why I was really surprised about what I found when I dissected some basal Hymenoptera specimens, specifically Athalia rosae (Tenthredinidae), Macroxyela ferruginae and Xyela sp. (Xyelidae). The second axillary is composed of two sclerites! In Figs 2–3, the second axillary is composed of a dorsal (2axd) and an elongate ventral (2axv) sclerites (2axd has a darker coloration on Fig 2 because the ventral wing layer is overlapping it in this image).
This finding raises numerous questions:
- How does this phenotype impact wing kinematics?
- Are there more Hymenoptera taxa with unfused second axillary and does the fusion of the two sclerites occurred once or multiple times in the course of Hymenoptera evolution?
- What about other insect taxa? Is the second axillary fused in all other Neoptera?
I really think that we will have lots of fun when we try to answer these questions and gather more information about the Hymenoptera wing base during our research, especially that these days we are able to apply some really new morphology techniques including X-ray- and CLSM-based 3D reconstructions and finite element analyses. Stay tuned for more results!
Update: You can now cite this as:
Mikó I, Deans AR. (2014) The second axillary in Hymenoptera. PeerJ PrePrints 2:e428v1 DOI: 10.7287/peerj.preprints.428v1
Leave a Reply