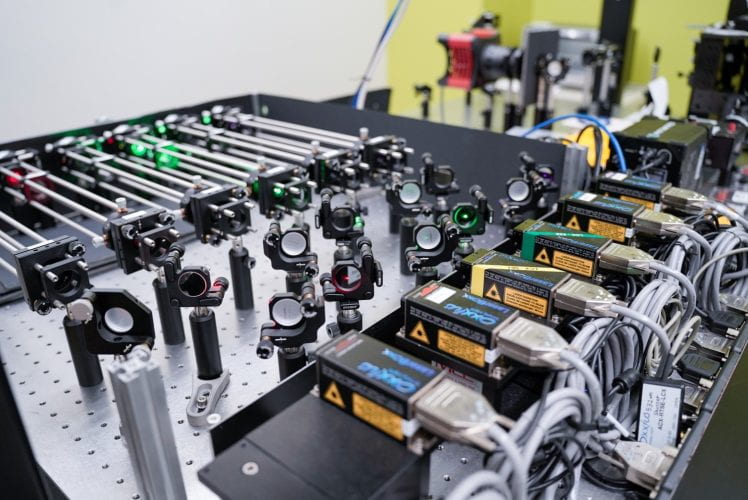
Dr. Daguan Nong is building the SCATTIRSTORM microscope from bolts and nuts!
And here is how it looks when it is finished!
BUT, what is this, and how it works?

When attempting to detect small objects that do not radiate light, we rely on detecting either their scatter or shadow.

In most cases, the shadow appears faint. (Left: scatter/shadow of cellulose. Right: cellulose becomes vivid and bright after being labeled with S4B and being excited.

We employed Total Internal Reflection Fluorescence Microscopy (TIRFM) to separate the excitation from the emission. This separation was achieved by taking advantage of the wavelength difference between excitation and emission caused by a Stokes shift resulting from the energy ‘lost’ during electron excitation and relaxation.

We require a fluorescent probe for TIRFM; however, for IRM, it is unnecessary since anything will inherently scatter something! And when the scatter meets the reflection, it Interference and reinforces!

![]()
Now, let’s shine the shadow and trace the light! (TIRFM images of Qdot-labeled enzyme Cel7a are superimposed with the IRM images of label-free cellulose. Each Cel7a molecule is tracked with nanometer precision by fitting its intensity with Gaussian curves)

And yes, we have an excellent way to stabilize the stage when tracking those enzymes!

And we even consider the enzymes’ equality as they work leisurely on the substrate by providing them with a homogeneous illumination. (90% of them are pausing, and the other 10% pause 90% of the time while they are moving)
Relavant publication:
https://opg.optica.org/boe/fulltext.cfm?uri=boe-12-6-3253&id=450922





