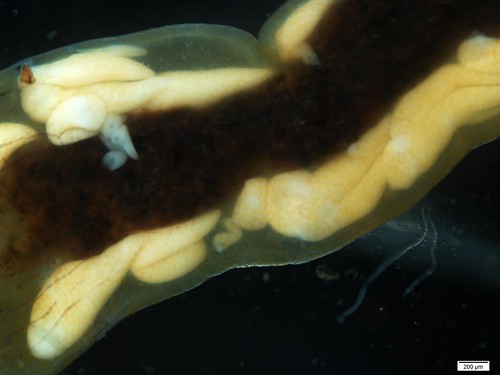
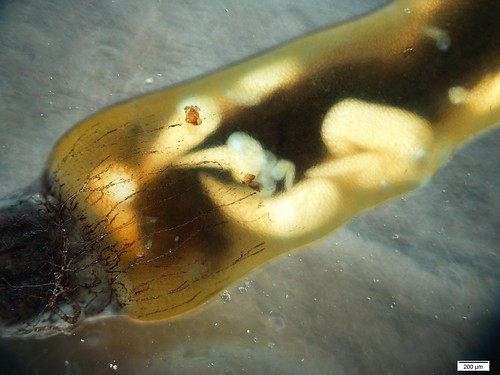
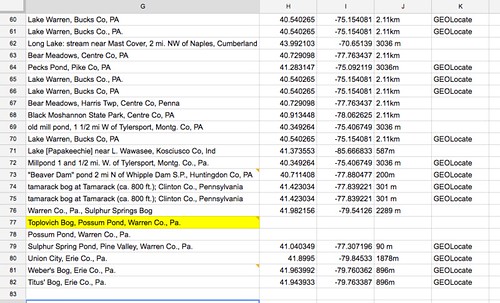
Excuse the clickbait title, but you’ve got to see this! For the last few weeks I have been slowly chipping away at digitizing and updating the Odonata ethanol collection. There is an abnormal smell that I expect can only come from 50-year-old ethanol with rubber tops, but I am glad that these little guys can be put in new homes- with a plastic top! Rafa did a great job with the louses and now I am trying to do the same with the odes.