The Facility’s PAGE equipment is free to use. If you don’t know how to run a gel, Tania can help!
Category Archives: Sample preparation questions
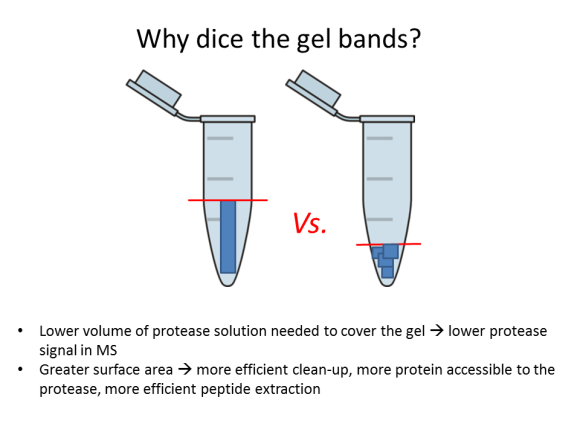
FAQ: Why should I dice gel bands?
Biotyper sample submission guidelines
The list of supplies and basic protocols for Biotyper can be found at the end of this post. In addition, the Biotyper manual in pdf format is available upon request. The Facility does not provide any of the supplies on this list and can only accept MS-ready target plates. For a demo on preparing the target plates, contact Tatiana.
To expedite your analysis, please fill out the Excel Sample ID Template with your sample names exactly as shown. All missing or incorrectly filled sample names will be replaced by the analysis date and your initials. You are responsible for keeping a record of your sample positions. The Excel file with the sample IDs must be emailed to Tatiana. Please attach any other information as separate files or documents.
Tips for successful microorganism identification
It is very important to exactly follow the procedure and use only the fresh, specified-grade reagents and solutions. MALDI is a competitive ionization process and is very sensitive to contaminants commonly present in histology-grade solvents and low-purity chemicals.
More is not better. A correct ratio of matrix to analyte is critical and achieved by following the procedure exactly.
Tube extraction (formic acid extraction) procedure requires extra few minutes of the prep time but yields much better results than the direct transfer procedure. Direct transfer procedure requires practice and is only applicable to non-spore-forming microorganisms. We recommend following the tube extraction procedure for all samples.
If you have any questions or suggestions or would like to discuss your project details, please email or call Tatiana.
Shipping the target plates
Plates can be shipped overnight on wet ice packs. To protect the sample spots, place the target plate facing down into its original box. You can tape it, so it does not get dislodged during shipping.
Desalting intact proteins for the direct infusion ESI MS
Electrospray ionization (ESI) and matrix-assisted laser desorption ionization (MALDI) are both competitive ionization processes in which a more abundant and/or more readily ionizable species will always win. Unless you are studying ionization of Tris, PEG, EDTA or CHAPS in the presence of small amounts of protein, the protein solution submitted for MS analysis should be essentially free of salts, detergents, chaotropic reagents, glycerol and other uninteresting interferences.
“So why not take a 10 mg/mL protein solution in a ‘mild buffer’ and dilute it to the required 0.1 mg/mL; the buffer concentration will be 100 times lower, and it is as good as desalted, right?” Wrong and here’s why:
Dilution is not a solution
Let’s say you have a 10 mg/mL solution of a 10 kDa protein in 10 mM Tris buffer, ‘mild’ enough. This means, you have a 10-fold molar excess of Tris compared to the protein. No matter how much you dilute this solution, the molar ratio of Tris to protein will remain 10:1 while it should be 1:50 or less.
For practical purposes, ‘essentially salt-free’ means that the total molar concentration of interfering species is much lower than that of the protein, e.g. at least 50-fold lower.
Clean-up strategies
There are many ways to clean up a protein solution. In each case, the choice of a protocol will depend on the properties of interfering species and on the size and properties of the protein. For example, detergents can be removed using specialized detergent removal spin columns. PEGs and other charge-neutral contaminants can be washed away by loading the protein onto an ion exchange medium, eluting the protein with a high-salt buffer, and desalting the eluate. Buffer salts, urea, and other small molecules can be removed by either a desalting spin column packed with size-exclusion resin or a centrifugal filter with an appropriate molecular-weight-cut-off (MWCO) membrane. If you need an advice on your protein sample clean-up, I am always happy to help; you know where to find me!
Desalting using centrifugal filters
Centrifugal filters with different MWCO membranes are probably the easiest way to desalt a small volume of protein solution, although there are a few caveats. For example, the regenerated cellulose Ultracel membranes might retain cellulose-binding proteins; in this case, the low protein binding Omega membranes from PALL would be a better choice.
How many spin-dilute cycles are enough to ‘completely’ desalt a protein solution? This is one of those annoying situations where the old trusty triplicate doesn’t always work. Here’s my handy formula to help me decide when to stop desalting and start analyzing:
Example 1.
Let’s use our 10 mg/mL 10 kDa protein in 10 mM Tris buffer and a 3 kDa MWCO Amicon centrifugal filter as an example. The filter has a 0.5 mL capacity, and we can load 50 uL of the protein solution and dilute it with 450 uL of water. After a 30-min centrifugation, the volume is 50 uL, but the concentration of Tris is now 10% (or 0.1) of the initial while the protein concentration is essentially unchanged. So our D, dilution factor for Tris after each dilution+spin is 0.1; our goal, M final is 0.01 mM Tris (100:1 protein:Tris); and our M initial is 10 mM Tris. To find the number of dilute+spin cycles, dust off your scientific calculators:
 After 3 dilute/spin repeats, we will achieve our acceptable concentration of Tris, 100 times lower than the protein concentration.
After 3 dilute/spin repeats, we will achieve our acceptable concentration of Tris, 100 times lower than the protein concentration.
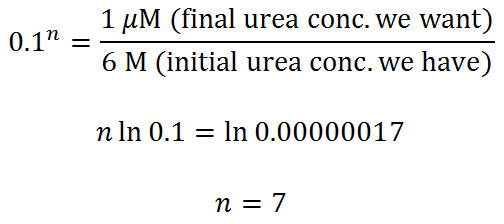
Example 2.
Let’s throw in some urea, dilute the protein to 0.1 mM, and do our calculation again. How many steps (n = ?) do we need to get from 6 M to 1 uM urea (100:1 protein-to-urea ratio) using the same spin column as before?
You probably can get away with 6 dilute/spin repeats, but 7 would be better.
For those of you who hate equations, here’s a picture that illuminates the desalting process using the first example.
“What if my protein is unstable without a buffer?”
In that case, it may not be amenable to the direct ESI MS. However, before throwing up your hands, try to desalt your protein. It might be stable in water at micromolar concentration long enough to be analyzed – I am speaking from experience. You also have an option of using LC ESI MS or submitting your partially desalted protein for MALDI TOF MS.
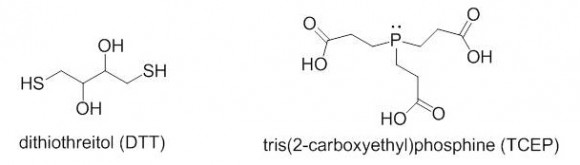
TCEP or DTT?
Which reagent is better for reducing disulfides, DTT or TCEP? The two reagents are quite different in their reactivity, stability towards oxidation, reaction mechanisms, and other categories.
DTT is a thiol-containing reagent, and this must be considered in applications involving thiol labeling. TCEP is charged in solution and should not be used in isoelectric focusing. Aqueous solutions of TCEP are quite acidic (pH 2-3).
TCEP HCl is odorless, air-stable crystalline solid, soluble in water at a > 1 M concentrations. It reduces disulfides at room temperature in < 5 min in dilute solutions (5 -50 mM). There is no need to remove TCEP prior to the use of sulfhydryl-reactive labels or crosslinkers. TCEP is selective toward disulfides, and is reactive at a broad pH range.
For those who love Chemistry:

Reduction of disulfide with TCEP. First step is rate-determining, kinetic rather than thermodynamic control.
You can learn more about DTT and TCEP from this article: “A Comparison between the Sulfhydryl Reductants Tris(2-carboxyethyl)phosphine and Dithiothreitol for Use in Protein Biochemistry”, Elise Burmeister Getz et al. Analytical Biochemistry 273, p. 73–80 (1999)
2D PAGE in less than one page
Approximate timeline
Day 1
Immobilized pH gradient (IPG) strip rehydration: active time 5 min per sample, rehydration is carried out overnight
Day 2
Isoelectric focusing (IEF): active time 5 min per sample, IEF 5-6 hrs.
Preparation for the 2nd dimension SDS PAGE: 1 hr.; necessary reagents and solutions are prepared during the last hour of IEF.
SDS PAGE, staining, and de-staining: 2.5 hrs. with de-staining overnight.
Brief procedure (learn more in the 2D PAGE manual )
Day 1 (late afternoon)
Protein sample should be lyophilized or precipitated. To keep the ionic strength of the protein solution at minimum, avoid salts. Non-ionic and zwitterionic solubilizing agents could be present, but keep in mind solubility limits.
Protein load for a complex sample to be stained with Coomassie is approximately 100 ug. The protein load varies depending on many factors including stain sensitivity, IPG range, or downstream applications (refer to the manual).
Protein sample is dissolved in the sample buffer (Bio-Rad cat #163-2106), 125 ul for 7 cm IPG strips and 185 uL for 11 cm IPG strips. The protein solution is pipetted into a channel in a rehydration tray. An IPG strip is placed gel side down into the channel, covered with mineral oil, and left overnight at 4 C.
Day 2 (early morning)
IPG strips are placed into a focusing tray, covered with mineral oil, and the tray is placed into the IEF cell for 5-6 hrs.
After the IEF is complete, strips are soaked in a reducing buffer, followed by alkylating buffer, rinsed in SDS running buffer, and placed at the top of an SDS-PAGE gel. A molten agarose solution is applied to the well. Once agarose solidifies, the gel is ready for the 2nd dimension electrophoresis.
After completion of the SDS-PAGE, gels are rinsed, stained, and destained. Alternatively, the gels can be electro-blotted.
Supplies
Immobilized pH gradient (IPG) strips
What IPG strip is the best for your experiment? Start by choosing the length (7 cm or 11 cm). Short strips are compatible with the Mini-PROTEAN cell, longer strips are compatible with the Criterion cell. All parameters being equal, a larger gel affords better resolution. Next select pH range depending on a type of your experiment: broad range (e.g. 3-10) for a global view, narrow range for a zoom-in view.
Bio-Rad cat #163-2000, 7 cm, pH 3–10, immobilized pH gradient (IPG) strip for first-dimension separations, pkg of 12
Bio-Rad cat #163-2014, 11 cm, pH 3–10, immobilized pH gradient (IPG) strip for first-dimension separations, pkg of 12
Buffers and gels
Rehydration/sample buffer, Bio-Rad cat #163-2106
Agarose, Bio-Rad cat #163-2111
Criterion Tris-HCl Gel, Bio-Rad cat #345-0040, Pkg of 1, 8–16% polyacrylamide gel, prep+2 well, 800 μl, 13.3 x 8.7 cm (W x L), for use with Criterion and Criterion Dodeca cells
10x Tris/Glycine/SDS, Bio-Rad cat #161-0732, 1 L, 10x premixed electrophoresis buffer, contains 25 mM Tris, 192 mM glycine, 0.1% SDS, pH 8.3 following dilution to 1x with water
SDS equilibration buffer II: 6M urea, 2% SDS, 0.375 M Tris-HCl (pH 8.8), 20% glycerol, Bio-Rad cat #163-2108
And for the best results: Electrode Wicks, Bio-Rad cat #165-4071, Pkg of 500, precut electrode wicks, for use with the PROTEAN® i12™ IEF system. (I still have about 450, no need to buy)
2D PAGE detailed procedure for the Bio-Rad starter kit
Please let me know if you are interested in a 2D PAGE experiment!
In-solution digestion of proteins
Purified proteins or protein mixtures can be digested in solution if an additional separation step is undesirable or unnecessary.
Proteins in solution are usually denatured by boiling or using denaturing buffers. During this step, the disulfide bonds must be reduced, and the sulfhydryl groups must be alkylated to prevent the disulfides from re-forming. The protein samples are then incubated with trypsin for several hours, and the resulting peptides can be analyzed by MS.
Denaturing buffers contain chaotropic agents, salts, and detergents at concentrations that inactivate trypsin. Before adding trypsin, you should desalt your protein sample and remove detergents. There are a number of the detergent removal and desalting options: detergent removal spin columns, size-exclusion and MW-cut-off spin columns, ion-exchange membranes and resins, etc. Gel-assisted proteolysis is another option, but then it is not really an ‘in-solution’ procedure, although it does not involve electrophoresis. I thought I should mention it here in case it could be of interest to you.
You will need
Digestion buffer: 16 mg/mL ammonium bicarbonate in water
Reducing reagent: 30 mg/mL TCEP (~100 mM, Sigma C4706) or 15 mg/mL DTT (Sigma D0632 ) in digestion buffer. NOTE: The 30 mg/mL TCEP stock solution must be prepared in 16 mg/mL (~200 mM) ammonium bicarbonate to bring up its pH. The final concentration of TCEP in the digestion mixture should be 5-10 mM.
Alkylating reagent: 18 mg/mL iodoacetamide (Sigma I1149) prepared fresh in the digestion buffer
Proteomics grade trypsin (e.g. Sigma T6567-5x20UG or Thermo Pierce 90057, 5 vials x 20 ug lyophilized powder). Trypsin, 20 ug can be dissolved in 20 uL of 1 mM HCl or 50 mM acetic acid, pH ~ 3, aliquoted and stored at -20C (stock solution).
To prepare activated (or working) trypsin solution, dilute trypsin stock solution with digestion buffer 10-fold to 0.1 ug/uL concentration.
Procedure
Volumes are approximate, it is a sample procedure after all. Trypsin should not exceed 5% of the total protein, provided the protein concentration range is known.
Combine 15 uL digestion buffer, 3 uL reducing reagent, and up to 12 uL sample solution containing 0.025 – 10 ug protein (total volume 30 uL)
Denature/reduce at 50-60 C (TCEP) or in a boiling water bath (DTT) for 5 – 10 min, cool to r.t., spin down to collect the sample
Add 3 uL alkylating reagent and incubate in the dark at r.t. for 20 min
If protein sample contains detergents, salts, or chaotropic agents, perform buffer exchange after the alkylation using a 3,000 MWCO centrifugal filter. It will be impossible to remove detergents after the digestion; and most detergents are not compatible with LC MS analysis. You can find a list of MS-compatible detergents here.
Add 1-5 uL activated trypsin and incubate at 37 C for 3 hrs. Optional: add 1-5 uL of fresh activated trypsin and incubate for an additional 2 hrs at 37 C or overnight.
Once the incubation is complete, the peptides can be submitted for analysis or stored at -20 C.
Helpful tips
Minimum sample amount required for MS analysis is in the fmol/uL range (ng/uL). Solutions of peptides at very low concentrations (e.g. less than 100 fmol/uL) should not be stored for more than 1-2 days.
Always run a control along with your sample. It could be a 1 mg/mL solution of bovine serum albumin or other standard protein that you have in your lab prepared in the same buffer as your sample and taken through the entire procedure. We don’t charge for analyzing your controls.
TCEP is a great reducing reagent because it does not contain -SH groups and thus does not consume iodoacetamide during alkylation, unlike DTT. TCEP solutions in water are acidic. Depending on your buffer composition, you might observe your sample coming out of the sample tube as a soapy foam the moment you add TCEP. It is pretty much impossible to put that foam back into the tube, don’t ask me how I know. So, prepare your TCEP solutions in ammonium bicarbonate buffer to get a pH close to 8. Another note about TCEP: it should never be stored in phosphate buffers because it quickly decomposes in the presence of phosphate.
Have I missed anything? Let me know!
Proteomics sample volume and concentration
Every sample is different in terms of purity and structural and compositional complexity. The MS detection sensitivity of a routine analysis is in a range of 0.1 to 10 pmol of protein. Some peptides ionize and/or fragment more efficiently and will produce good spectra at 0.1 pmol per injection while other peptides may be completely ‘invisible’ even at 100 pmol per injection. Keep in mind that it is always easier and faster to dilute a sample than to concentrate it.
For routine analyses, a 1-microliter injection is usually made unless the protein concentration is known and requires a larger-volume injection. The injection volume cannot exceed 6 microliters.
For simple mixtures (in-solution digested purified or enriched proteins and in-gel digested protein bands), 3 to 10 microliters of sample must be submitted because smaller volumes tend to dry out. This is only true of expertly prepared samples that do not require purification and/or filtration.
If you are planning to analyze a complex mixture, please contact Tatiana.
Ammonium bicarbonate or triethylammonium bicarbonate?
This was a question from one of my blog’s secret readers. Actually, most of the time I feel like I am talking to myself: “Hey Tania, how do you prepare a protein sample for proteolysis?” “Well, Tania, let me show you in a step-by-step tutorial.” No comments, no questions, no pointing out typos, no “thank you, Tania, but there’s a better way to do this”?
Oh well, back to ammonium bicarbonate. This is a volatile salt which breaks down to ammonia, carbon dioxide, and water. Volatile salts are the only salts compatible with MS. Aqueous solutions of ammonium bicarbonate (0.01 – 0.1 M) have pH around 8, the optimal pH for trypsin activity. Ammonium bicarbonate competes with basic amino acids for Coomassie dye, which makes it a great de-staining reagent for the in-gel digestion procedure. All this goodness comes at a very reasonable price – what not to like? Another ammonium salt, triethylammonium bicarbonate (TEAB), is more volatile than ammonium bicarbonate; it is also more expensive. TEAB is a buffer of choice for LC-MS applications: TMT (iTRAQ) amine-reactive labeling, ion-exchange chromatography, protein solubilization (when neutral and acidic pH is undesirable), in-gel digestion, etc.
Detergent removal 2: Affinity resin
To continue our detergent theme, here’s an affinity-based detergent removal method that is faster than the gel-assisted proteolysis but removes only the detergents, leaving the salts and chaotropic agents for you to deal with later. Unlike the gel-assisted method, it works for both proteins and peptides. The gel-assisted method is best suited for proteins, because the small, more soluble peptides are likely to elute out of the gel matrix during the washing.
I use Pierce detergent removal spin columns (0.125 mL format) in my lab, but there are other options available such as Bio-Beads or HyperD, each with its own pros and cons in the business of detergent removal. Pierce also sells a so-called HiPPR detergent removal resin (high protein and peptide recovery) for low-protein-concentration samples. The initial % detergent in such samples must also be low (ca. 1%).
The Pierce resin removes common ionic, nonionic, and zwitterionic detergents from protein and peptide solutions. This oligosaccharide-based affinity resin has a small hydrophobic cavity which creates a microenvironment for a detergent’s nonpolar moiety to enter and form an inclusion complex.
The workflow is simple: (1) centrifuge the column to remove storage buffer, (2) wash 3 times with your favorite buffer (pH 5-10), discarding the buffer each time, (3) add protein or peptide solution and let the resin do its magic for 2-5 min at room temperature, (4) centrifuge to collect your >95% detergent-free sample, i.e. don’t discard the flow-through this time!
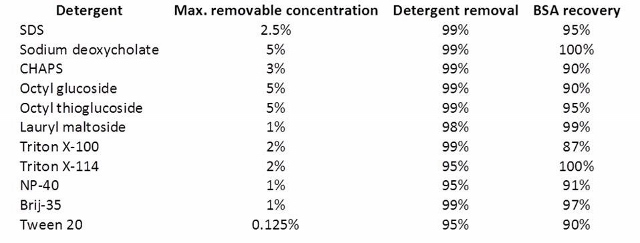
Antharavally and co-workers from Thermo Scientific Pierce Protein Research published a study examining the detergent removal efficiency and protein recovery using their resin under several conditions (doi:10.1016/j.ab.2011.05.013). A table from this reference gives you some idea of the detergent concentrations removable with the Pierce resin.
Samples (0.1 ml containing 0.100 mg BSA + detergent at maximum concentration) were processed through 0.5 ml of Pierce detergent removal resin, and the residual detergent was measured as described in Materials and Methods. Protein concentration was determined by BCA protein assay (Pierce).